Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system
Introduction
Chemotherapy is a major method for treating advanced malignant tumors in the digestive system (1-3). When drugs are administered intravenously, a small amount infiltrates the tumor tissue, while a large amount is delivered to the normal tissues of the body through the blood circulation, thus leading to adverse reactions (4,5). One of the most important factors limiting the effectiveness of chemotherapy is the primary and secondary resistance of cancer cells (6,7). Ultrasound sonoporation is a new method of local drug delivery which has been applied in medical research due to its noninvasiveness, local applicability, and proven safety as an ultrasonographic imaging technique (8,9). This method restricts the release of the drugs to a focal ultrasound zone and enhances the effect of chemotherapy in sonoporated areas (10,11). Microbubbles thus offer considerable promise as a means of improving the therapeutic efficiency of chemotherapy, as well as decreasing toxicity to healthy tissues (12,13).
The mechanism by which sonoporation enhances the sensitivity of chemotherapy relies on a cavitation effect and a mechanical effect (14-16). The sonoporation effect occurs by means of the characteristics of microbubbles under different mechanical indexes (MIs) (17-19). Clinically applied microbubbles are 2−8 mm bubbles composed of sulfur hexafluoride gas encapsulated by phospholipid. The microbubbles are stable under a low MI, which is applied during imaging. Under a high MI, microbubbles injected into the blood circulation undergo a series of dynamic processes, such as oscillation, expansion and contraction, and release mechanical energy, such as shockwaves and microjets, at the moment of bubble cavitation (20,21). As tumor neovascularization is incomplete, weak and highly permeable, mechanical energy can directly cause formation of transient pores thus increasing the permeability of the cell membrane, which is known as the sonoporation effect (22-24). The sonoporation effect has been demonstrated to significantly increase the penetration of chemotherapeutic drugs into tumor cells and increase the drug concentration (25,26). In addition, more drug binding sites can be exposed to increase the sensitivity of the tumor to chemotherapy (27).
At present, research is mainly at the stage of animal experimentation (28-30). However, clinical studies on the use of ultrasound with microbubbles are few. Kotopoulis et al. took the lead in performing a clinical trial of ultrasound combined with microbubbles for enhancing the chemotherapeutic sensitivity of pancreatic cancer (31). As the current clinical studies were at the primary stage of research regarding ultrasound combined with microbubbles, this study mainly focused on the safety of this technique for enhancing the chemotherapeutic sensitivity of digestive malignancies to lay the foundation for further studies of effectiveness.
Materials and methods
This study was registered in Clinicaltrials.gov (No. NCT02233205), and was approved by the Ethical Committee of Peking Unicersity Cancer Hospital. All the patients signed informed consent before they were enrolled in the study. The detailed schedule of this study is shown in Table 1.
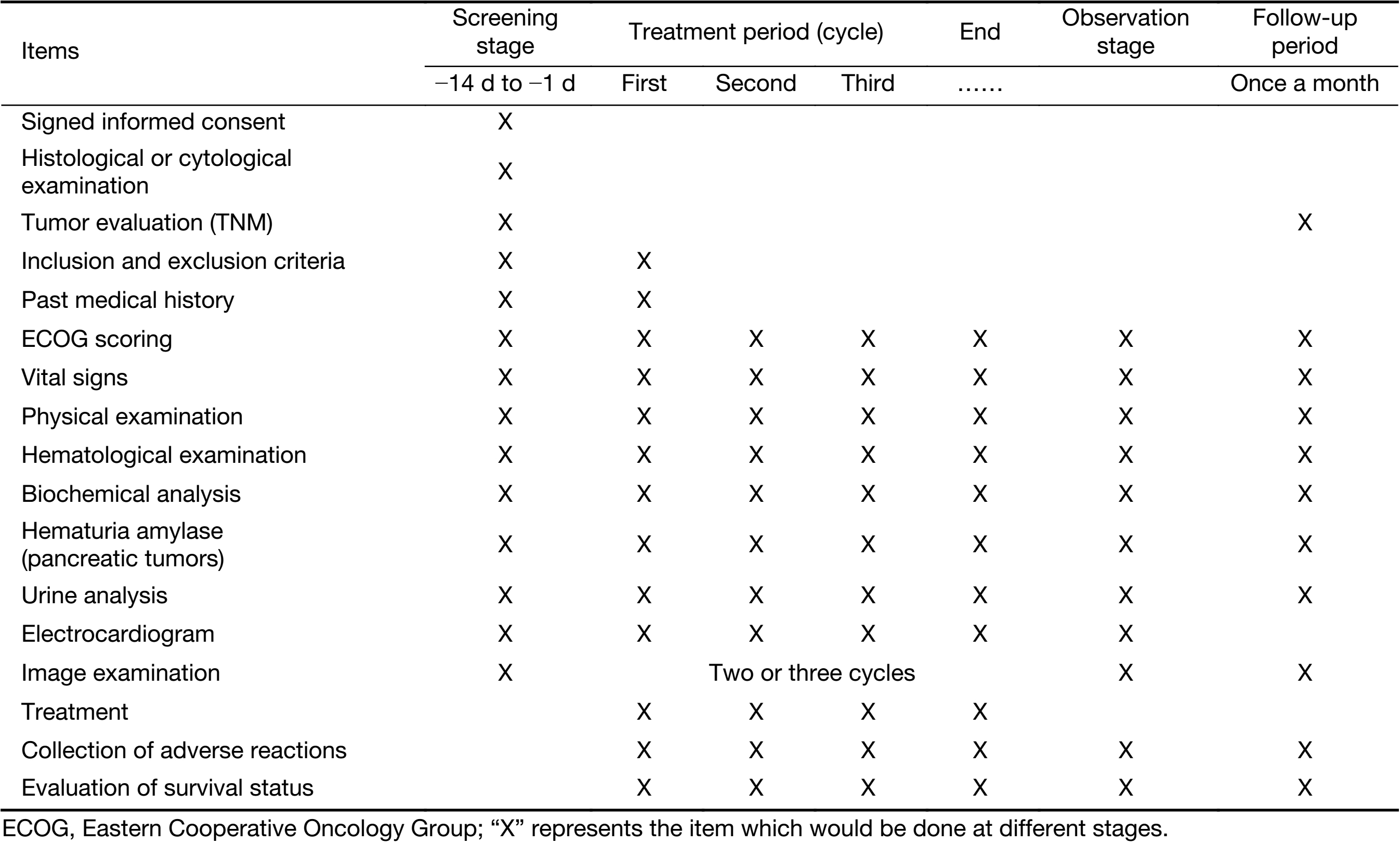
Full table
Study subjects
The study group comprised patients with liver metastases from malignant tumors of the digestive system (gastric cancer, colon cancer, etc.) and patients with pancreatic cancer. They all had previously failed routine chemotherapy, volunteered to participate in the study and met the inclusion criteria. The inclusion criteria were as follows: 1) age between 18−75 years old; 2) male or female; 3) histological or cytological diagnosis of liver metastasis from a malignant tumor of the digestive system (gastric cancer, colon cancer, etc.) or from pancreatic cancer; 4) previous failure of routine chemotherapy; 5) measurable and evaluable tumor lesions on images [using enhanced computed tomography (CT) or magnetic resonance imaging (MRI)]; 6) physical status Eastern Cooperative Oncology Group (ECOG) score ≤2; and 7) expected survival of more than 12 weeks.
Eighteen study subjects were originally identified for inclusion. Fifteen patients were classified into five groups (groups A, B, C, D and E), and each group contained three patients. If serious adverse reactions occurred, another three patients were included. The first four groups were classified according to MI from low to high. Group E had the same MI as group D, but had double the treatment time of ultrasound combined with microbubbles. As patients after chemotherapy often had multiple adverse reactions and physical decline, the longer treatment time with ultrasound combined with microbubbles was clinically unrealistic, and three patients in the last group (group E) were excluded. Between October 2014 and June 2016, 12 patients met the enrollment criteria and completed the follow-up (Figure 1).
Ultrasonographic machine and contrast agent
A LogiQ E9 ultrasonic diagnostic apparatus and C1-5 abdominal convex probe (GE Healthcare, Milwaukee, Wisconsin, USA) were used. Sonovue (Bracco, Milan, Italy) was used as microbubbles. Lyophilized SonoVue powder was dissolved in 5 mL of saline. Two milliliters of the suspension was injected into the antecubital vein via a 20-G cannula within 2−3 s, followed by a 5 mL saline flush.
Treatment methods
According to the patient’s history and condition, appropriate chemotherapy regimens were determined by experienced physicians in the Department of Gastrointestinal Oncology. All the second-line chemotherapy regimens, dosages and cycles are shown in Table 2. All chemotherapy regimens were administered intravenously.
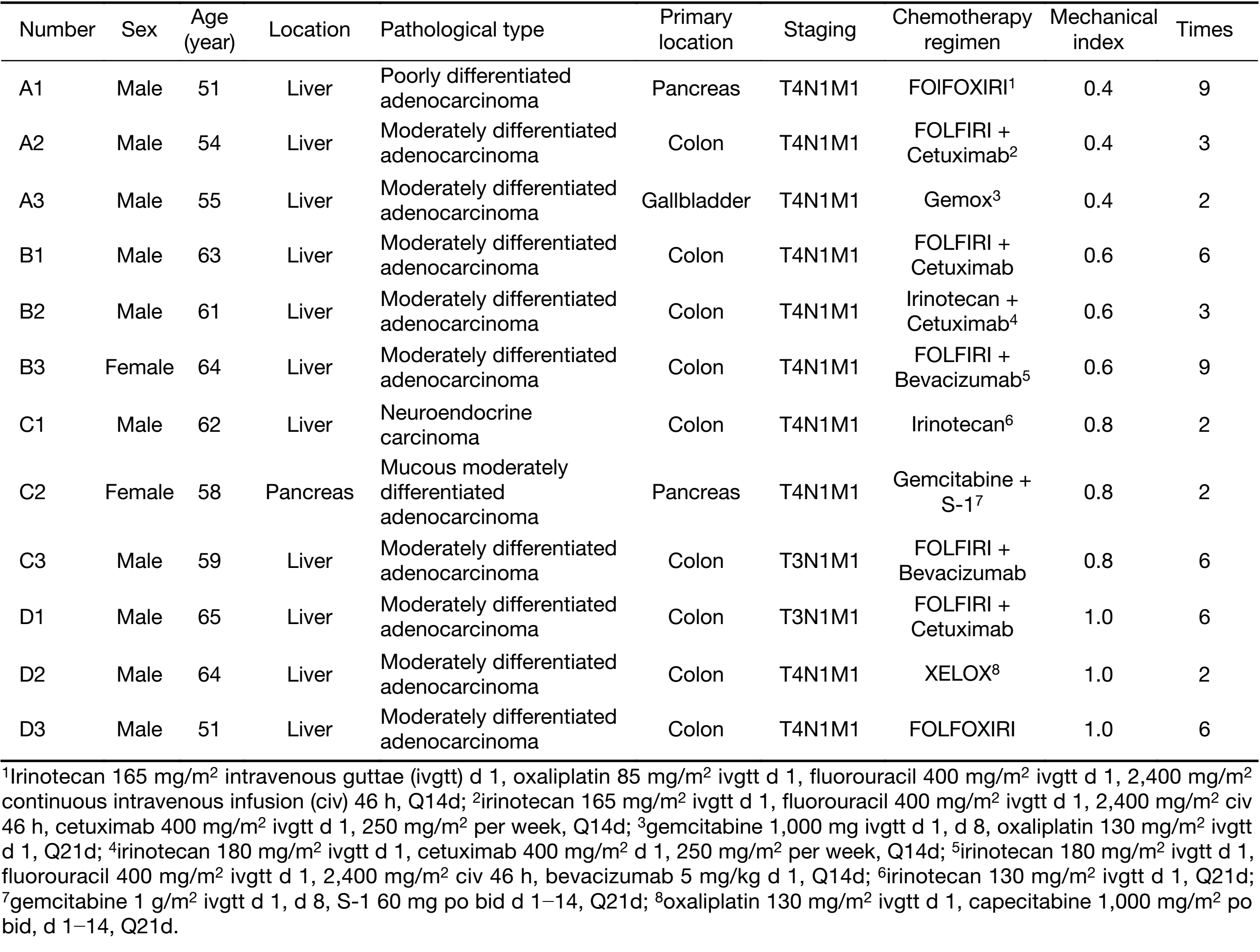
Full table
With half an hour after intravenous chemotherapy, the patients came to the Department of Ultrasound to receive treatment of ultrasound combined with microbubbles. First, the tumor was located in the center of image by transcutaneous ultrasound through the abdominal wall. Then, contrast mode was entered. One milliliter contrast agent suspension was injected through a vein. The perfusion time for the microbubbles was set to 6 s. Then, contrast mode was exited. The breaking time for the microbubbles was set to 4 s. One milliliter of contrast agent suspension was injected every 4 min. The perfusion and breaking processes were repeated. A total of 5 mL of contrast agent suspension was injected. After the end of treatment, the patients returned to the ward when no obvious discomfort was observed.
Energy increasing principle
The ultrasonic energy design used the traditional Modified Fibonacci dose increasing method. The primary MI of Group A was set at 0.4 according to experience of Kotopoulis et al. (31). The MIs of the other three groups were 0.6, 0.8 and 1.0, respectively. There were 3 patients in each group at first. If no serious adverse reactions occurred in the group, the experiment was performed on the next group. If 1/3 of patients (1 patient) in a group experienced serious adverse reactions, 3 patients were added to the group (for a total of 6 patients in the group). If the three additional patients had no serious complications, the next group of experiments was performed. If ≥1 patient among the three added patients or ≥2 patients among the total 6 patients had serious adverse reactions, the maximum energy tolerance level was determined to be reached. Once the maximum tolerance level was reached, the energy level was not increased. In addition, another 3 patients were included in a group to evaluate the energy level just below the maximum tolerance level. If one patient among those three patients had a serious adverse reaction, the main investigator decided whether to stop the study.
Clinical evaluation and follow-up
Safety evaluation
Safety evaluations included physical examinations, assessment of vital signs, physical status scoring, routine hematology and biochemical laboratory tests, assessment of hematuria and amylase levels, evaluation of electrocardiography (ECG) changes, etc. The safety assessment included observing and recording all adverse events, which were graded from 0−4 according to the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE V4.03). Adverse events included pain, loss of appetite, fatigue, fever, nausea and vomiting, diarrhea, bloating, myelosuppression and neurotoxicity, etc.
Efficacy evaluation
The contrast-enhanced CT and MRI studies were used to assess the efficacy of ultrasound combined with microbubbles. The targeted tumors of each patient after treatment were evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) criteria: complete response (CR) was defined as the disappearance of all target lesions. Any pathological lymph nodes (whether target or non-target) must have reduced along their short axis to <10 mm. Partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of the target lesions, using the baseline sum diameters as a reference. Progressive disease (PD) was defined as at least a 20% increase in the sum of the diameters of the target lesions, using the smallest sum on record as a reference. Stable disease (SD) was defined as neither sufficient reduction in size to be defined as PR nor a sufficient increase in size to be defined as PD, using the smallest sum diameters on record as a reference.
Follow-up
Eleven patients withdrew from the study due to tumor progression, and one patient voluntarily discontinued treatment. All patients were followed up until PD occurred. If serious complications occurred, the patients were followed up until remission of these adverse reactions.
Results
Clinical data of enrolled patients
The 12 enrolled patients were divided into four groups according to MI, and each group had 3 patients. There were 10 males and 2 females. The median age was 60 (range, 51−65) years old. Eleven patients had hepatic metastases, among which 5 had a pathological diagnosis, and 6 had a clinical diagnosis. All 11 patients had a pathological diagnosis of the primary lesions. One patient was pathologically diagnosed with pancreatic carcinoma. A total of 56 treatments with ultrasound combined with microbubbles were performed. The detailed clinical data of the patients are shown in Table 2.
Adverse reactions of enrolled patients
According to the CTCAE V4.03, no serious adverse reactions occurred in all the patients included in the study. The pressure of the probe on the surface of the patients’ skin did not increase local pain. The adverse reaction rate is shown in Table 3. Of the adverse reactions recorded, level 2 bone marrow suppression occurred in one patient (2 times), and level 2 vomiting occurred in one patient (6 times). Other adverse events including loss of appetite, fatigue, fever, nausea and vomiting, diarrhea, bloating, myelosuppression and neurotoxicity were all level 1 adverse reactions. Among all the adverse reactions, the duration of bone marrow suppression lasted for less than two weeks and the duration of other adverse reactions lasted for less than one week. All the adverse reactions resolved with close observation or symptomatic treatment.
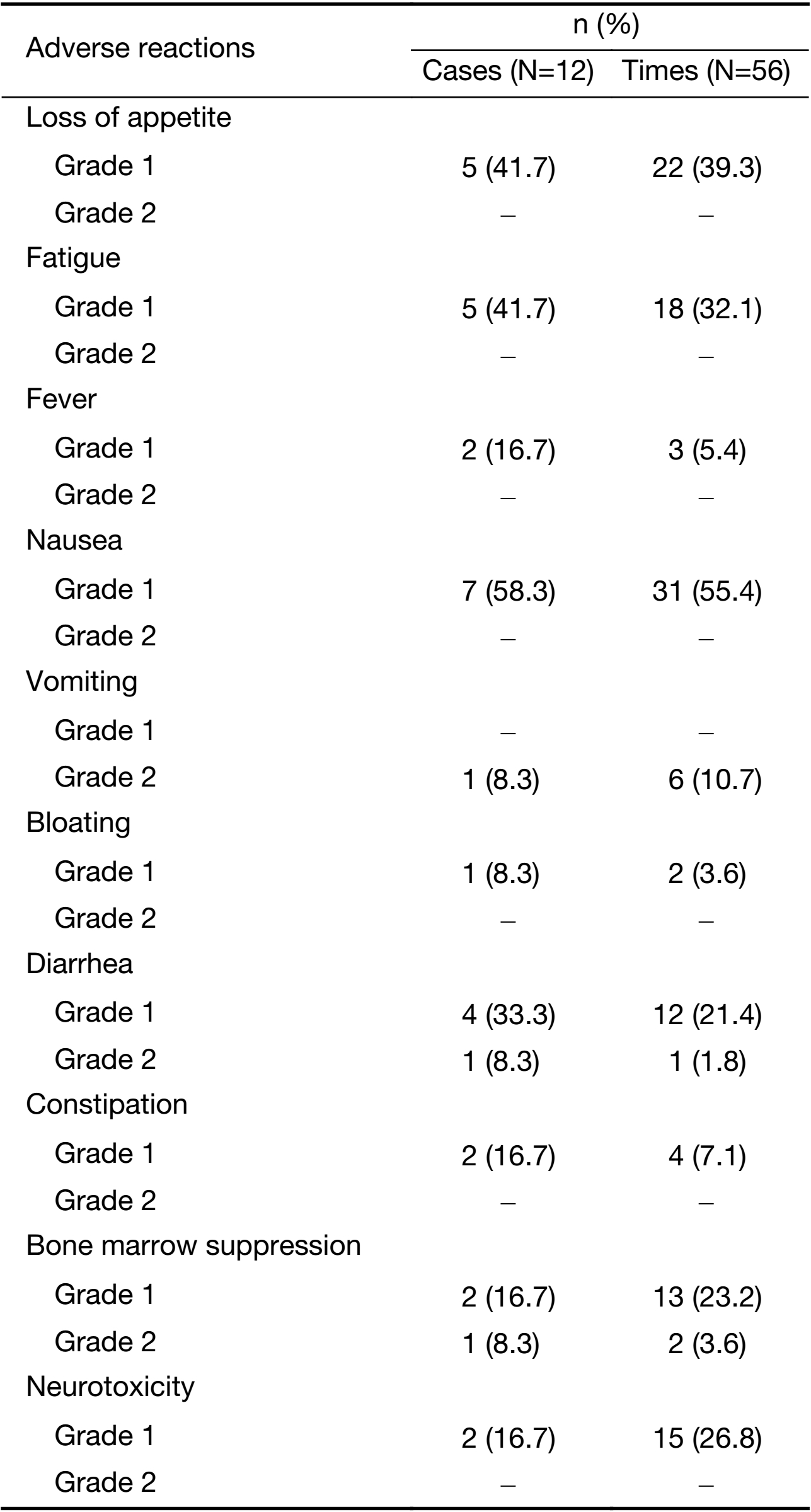
Full table
Apart from fever, the other adverse reactions are common adverse reactions of second-line chemotherapy for malignant tumors of the digestive system. In this study, level 1 fever occurred in 2 patients (3 times), which might be related to treatment with the combination of ultrasound and microbubbles.
Adverse reactions of enrolled patients with different MIs
The adverse reactions associated with different MIs are shown in Table 4. Among all the adverse reactions, fever, diarrhea, vomiting and bone marrow suppression resolved after symptomatic treatment. When the MI was 0.4, level 1 fever occurred in 2 patients (3 times), level 1 diarrhea occurred in 2 patients (6 times), and level 1 bone marrow suppression occurred in 2 patients (13 times). When the MI was 0.6, level 1 diarrhea occurred in 1 patient (1 time). When the MI was 0.8, level 2 bone marrow suppression occurred in 1 patient (2 times). When the MI was 1.0, level 2 vomiting occurred in 1 patient (6 times), level 1 diarrhea occurred in 1 patient (5 times), and level 2 diarrhea occurred in 1 patient (1 time). All the adverse reactions were grade 1 or 2. The severity of adverse reactions did not increase with increases in MI. Therefore, when the MI was no more than 1.0, treatment with ultrasound combining with microbubbles to enhance chemotherapeutic sensitivity was safe.
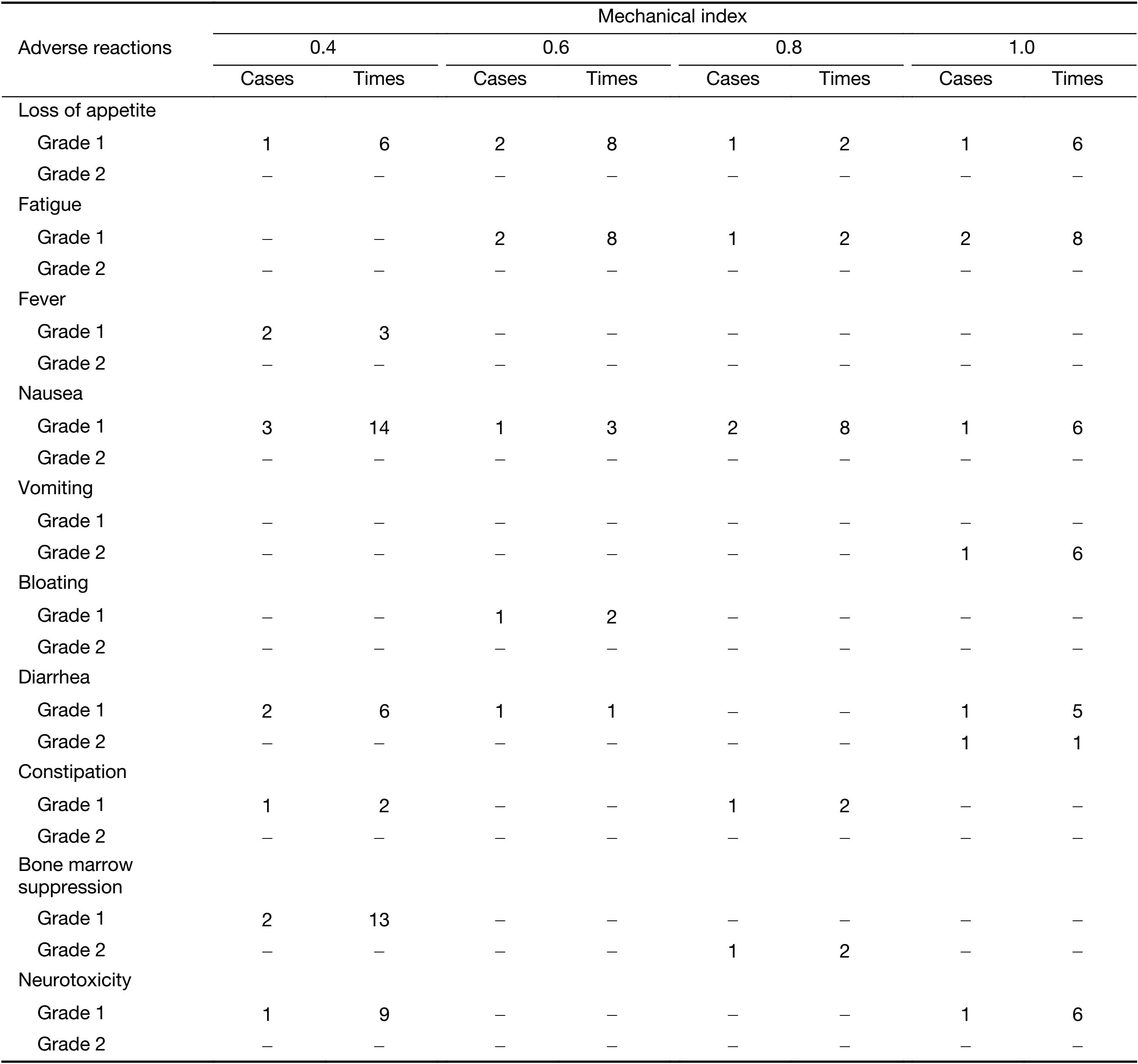
Full table
Therapeutic evaluation
The therapeutic evaluation of each treatment cycle and follow-up results after treatment are shown in Table 5. All the 12 patients were followed up until PD occurred. The median progression-free survival (PFS) was 91 (interquartile range, 88) d. Progression in all the patients was restricted to the originally affected organs, and infiltration into other tissues or organs did not occur.

Full table
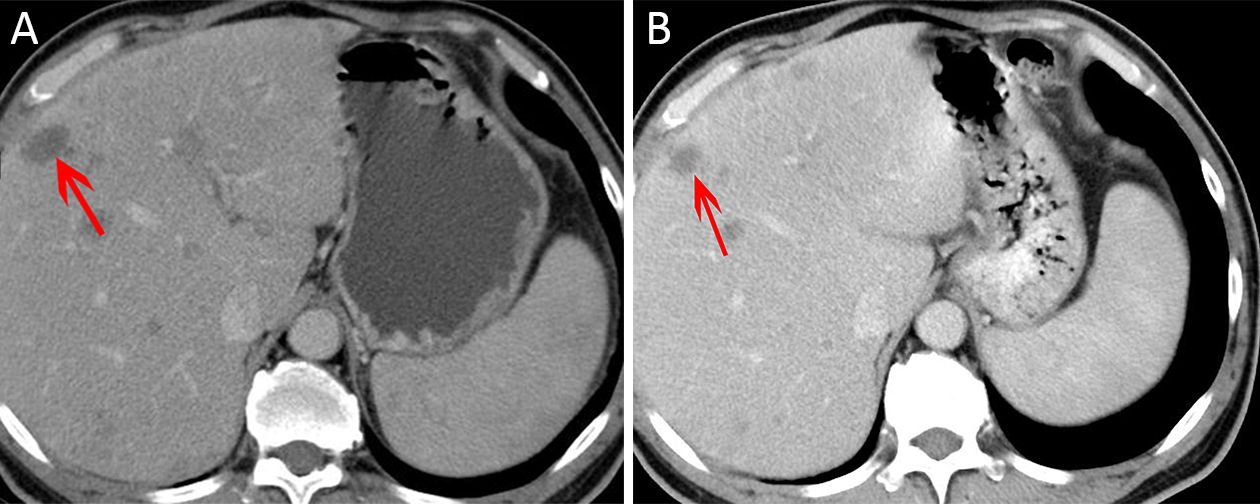
Six patients obtained SD, and one patient obtained PR after the first treatment cycle. When the MI was 0.4, one patient experienced good treatment efficacy, which was evaluated as PR once (Figure 2) and SD once, and his PFS was 168 d. When the MI was 0.6, 2 patients (3 times) had SD. When the MI was 0.8, 1 patient (2 times) had SD. When the MI was 1.0, 2 patients (4 times) had SD.
Discussion
The application of microbubble contrast agents in imaging has been widely clinically accepted (32,33). Currently, the combination of microbubbles with ultrasound has become a universal theranostic method (34,35). The sonoporation effect is the mechanical basis for the efficacy of this treatment (36,37). Sonoporation is defined as the interaction of ultrasound with ultrasonic contrast agents to temporarily permeabilize the cell membrane and allow the uptake of various substances, such as DNA, drugs, and other therapeutic compounds, from the extracellular environment (38-41). The role of the sonoporation effect in targeted drug release and gene therapy has been demonstrated in animal experiments (42-44). This technique is a promising approach for increasing drug and gene delivery efficiency (38,45-47). The primary objective of this study was to explore the safety of ultrasound combined with microbubbles in enhancing the chemotherapeutic sensitivity of malignant tumors in the digestive system. The secondary objective was to preliminarily evaluate the efficacy of this novel method.
This study subjects were patients with advanced malignant tumors of the digestive system. All the chemotherapy regimens were second-line chemotherapies. Other than fever, the other adverse reactions are common complications of second-line chemotherapy. According to the CTCAE V4.03, all the adverse reactions were grade 1 or 2 and resolved after symptomatic treatments. No serious adverse reactions occurred in any of the patients included in the study. In a study by Kotopoulis et al. (31) performed in Norway, treatment with ultrasound combined with microbubbles was shown to enhance the chemotherapeutic efficacy of gemcitabine; 5 patients received treatment 10−27 times and did not experience any increased discomfort with this treatment compared to treatment with chemotherapeutic gemcitabine alone. The study by Dimcevski et al. (48) reported that treatment with ultrasound combined with microbubbles and gemcitabine did not increase patient discomfort compared to treatment with gemcitabine alone. In our study, although fever occurred in 2 patients, they both were grade 1 and resolved with symptomatic treatment. Observing the adverse reactions among the different MIs, the extent and duration of the adverse reactions did not increase with increases in MI. All the patients experienced progression in situ, and no new lesions appeared when the patients were followed up until PD occurred. Therefore, when the MI is no greater than 1.0, treatment with ultrasound combined with microbubbles for enhancing chemotherapeutic sensitivity is safe.
Among the 12 patients, one patient with an MI of 0.4 obtained a good curative effect and reached PR after the first treatment cycle. Grade 1 fever (2 times) occurred in this patient, which might be related to the tumor necrosis after treatment. Therefore, an MI of 0.4 can be recommended for evaluation in further studies. An animal experiment by Lin et al. (49) reported that combining ultrasound and microbubbles was able to destruct tumor blood vessels and improve the penetration of liposomal adriamycin into tumor tissue in mice with subcutaneous tumors. The study by Kotopoulis et al. (31) reported that among 5 patients who received the combined treatment with ultrasound, microbubbles and gemcitabine, 2 patients showed significant tumor shrinkage, and 3 patients showed evidence of tumor growth inhibition. In addition, the patients in the study remained in good condition for a longer time than those in the control group. The study by Dimcevski et al. (48) reported that 5 of 10 patients showed a reduction in tumor size after treatment with ultrasound and microbubbles, which enhanced the chemotherapeutic sensitivity of gemcitabine, and the median survival was longer in this group than in the group of patients treated with gemcitabine alone (17.6 months vs. 8.9 months, P=0.011). As the chemotherapeutic sensitivities of malignant biliary and ampullary tumors are relatively worse than those of other malignant tumors in the digestive system, the application of ultrasound combined with microbubbles for enhancing the effectiveness of chemotherapy and prolonging patient survival is worth further study and discussion.
The primary limitation of this study was that historical control and random control cannot be formed as the patients’ diseases were multiple and the treatment regimens were not uniform. As this study mainly focused on the safety of ultrasound combined with microbubbles, random controlled study to explore effectiveness is needed for further studies.
Despite the many studies on ultrasound-mediated gene and drug delivery using microbubbles, the conditions under which ultrasound-mediated delivery is most effective have not been determined (50-52). Although this study identified a relatively good MI, the sample size was small for determining the ideal MI. In addition, there is no consensus on the ideal dosage of microbubbles used to enhance the efficacy of treatment. Further work should be done to determine ideal conditions for better therapeutic effects.
Conclusions
Our preliminary data showed that treatment of combining ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system was safe when the MI was no greater than 1.0. This study also showed the potential clinical application of the treatment of combining ultrasound and microbubbles.
Acknowledgements
This study was sponsored by National Key Research and Development Plan (No. 2017YFC0107300 and No. 2017YFC0107303).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol 2012;18:25–33. [PubMed] DOI:10.3748/wjg.v18.i1.25
- Zhou H, Song Y, Jiang J, et al. A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res 2016;28:598–605. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.06.06
- Liu F, Yang L, Wu Y, et al. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 2016;28:589–97. [PubMed] DOI:10.20524/aog.2016.0050
- Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther 2013;7:375–88. [PubMed] DOI:10.2147/DDDT.S31564
- Fiorentini G, Sarti D, Aliberti C, et al. Multidisciplinary approach of colorectal cancer liver metastases. World J Clin Oncol 2017;8:190–202. [PubMed] DOI:10.5306/wjco.v8.i3.190
- Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol 2014;20:9775–827. [PubMed] DOI:10.3748/wjg.v20.i29.9775
- Masui K, Gini B, Wykosky J, et al. A tale of two approaches: complementary mechanisms of cytotoxic and targeted therapy resistance may inform next-generation cancer treatments. Carcinogenesis 2013;34:725–38. [PubMed] DOI:10.1093/carcin/bgt086
- Leow RS, Wan JM, Yu AC. Membrane blebbing as a recovery manoeuvre in site-specific sonoporation mediated by targeted microbubbles. J R Soc Interface 2015:12. DOI:10.1098/rsif.2015.0029
- Lentacker I, Geers B, Demeester J, et al. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther 2010;18:101–8. [PubMed] DOI:10.1038/mt.2009.160
- Qin J, Wang TY, Willmann JK. Sonoporation: Applications for Cancer Therapy. Adv Exp Med Biol 2016;880:263–91. [PubMed] DOI:10.1007/978-3-319-22536-4_15
- Sennoga CA, Kanbar E, Auboire L, et al. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin Drug Deliv 2017;14:1031–43. [PubMed] DOI:10.1080/17425247.2017.1266328
- Lajoinie G, De Cock I, Coussios CC, et al. In vitro methods to study bubble-cell interactions: Fundamentals and therapeutic applications . Biomicrofluidics 2016;10:011501. [PubMed] DOI:10.1063/1.4940429
- Nejad SM, Hosseini H, Akiyama H, et al. Reparable cell sonoporation in suspension: theranostic potential of microbubble. Theranostics 2016;6:446–55. [PubMed] DOI:10.7150/thno.13518
- Wang YU, Chen YN, Zhang W, et al. Upregulation of ULK1 expression in PC-3 cells following tumor protein P53 transfection by sonoporation. Oncol Lett 2016;11:699–704. [PubMed] DOI:10.3892/ol.2015.3946
- Geers B, Lentacker I, Sanders NN, et al. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. J Control Release 2011;152:249–56. [PubMed] DOI:10.1016/j.jconrel.2011.02.024
- Helfield B, Chen X, Watkins SC, et al. Biophysical insight into mechanisms of sonoporation. Proc Natl Acad Sci U S A 2016;113:9983–8. [PubMed] DOI:10.1073/pnas.1606915113
- Zhao YZ, Du LN, Lu CT, et al. Potential and problems in ultrasound-responsive drug delivery systems. Int J Nanomedicine 2013;8:1621–33. [PubMed] DOI:10.2147/IJN.S43589
- Martin KH, Dayton PA. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2013;5:329–45. [PubMed] DOI:10.1002/wnan.1219
- Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol 2009;54:R27–57. [PubMed] DOI:10.1088/0031-9155/54/6/R01
- Fan Z, Chen D, Deng CX. Characterization of the dynamic activities of a population of microbubbles driven by pulsed ultrasound exposure in sonoporation. Ultrasound Med Biol 2014;40:1260–72. [PubMed] DOI:10.1016/j.ultrasmedbio.2013.12.002
- Tzu-Yin W, Wilson KE, Machtaler S, et al. Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr Pharm Biotechnol 2013;14:743–52. [PubMed]
- Forbes MM, O’Brien WD Jr. Development of a theoretical model describing sonoporation activity of cells exposed to ultrasound in the presence of contrast agents. J Acoust Soc Am 2012;131:2723–9. [PubMed] DOI:10.1121/1.3687535
- Fan Z, Kumon RE, Deng CX. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther Deliv 2014;5:467–86. [PubMed] DOI:10.4155/tde.14.10
- van Rooij T, Skachkov I, Beekers I, et al. Viability of endothelial cells after ultrasound-mediated sonoporation: Influence of targeting, oscillation, and displacement of microbubbles. J Control Release 2016;238:197–211. [PubMed] DOI:10.1016/j.jconrel.2016.07.037
- Shamout FE, Pouliopoulos AN, Lee P, et al. Enhancement of non-invasive trans-membrane drug delivery using ultrasound and microbubbles during physiologically relevant flow. Ultrasound Med Biol 2015;41:2435–48. [PubMed] DOI:10.1016/j.ultrasmedbio.2015.05.003
- Dixon AJ, Dhanaliwala AH, Chen JL, et al. Enhanced intracellular delivery of a model drug using microbubbles produced by a microfluidic device. Ultrasound Med Biol 2013;39:1267–76. [PubMed] DOI:10.1016/j.ultrasmedbio.2013.01.023
- De Cock I, Zagato E, Braeckmans K, et al. Ultrasound and microbubble mediated drug delivery: acoustic pressure as determinant for uptake via membrane pores or endocytosis. J Control Release 2015;197:20–8. [PubMed] DOI:10.1016/j.jconrel.2014.10.031
- Shen ZY, Shen E, Diao XH, et al. Inhibitory effects of subcutaneous tumors in nude mice mediated by low-frequency ultrasound and microbubbles. Oncol Lett 2014;7:1385–90. [PubMed] DOI:10.3892/ol.2014.1934
- Shen ZY, Xia GL, Wu MF, et al. The effects of low-frequency ultrasound and microbubbles on rabbit hepatic tumors. Exp Biol Med (Maywood) 2014;239:747–57. [PubMed] DOI:10.1177/1535370214525320
- Nomikou N, Feichtinger GA, Redl H, et al. Ultrasound-mediated gene transfer (sonoporation) in fibrin-based matrices: potential for use in tissue regeneration. J Tissue Eng Regen Med 2016;10:29–39. [PubMed] DOI:10.1002/term.1730
- Kotopoulis S, Dimcevski G, Gilja OH, et al. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys 2013;40:072902. [PubMed] DOI:10.1118/1.4808149
- Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol 2007;14:876–84. [PubMed] DOI:10.1016/j.nuclcard.2007.09.008
- Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol 2007;17:1995–2008. [PubMed] DOI:10.1007/s00330-007-0623-0
- Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res 2009;42:881–92. [PubMed] DOI:10.1021/ar8002442
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 2007;9:415–47. [PubMed] DOI:10.1146/annurev.bioeng.8.061505.095852
- Okada K, Kudo N, Niwa K, et al. A basic study on sonoporation with microbubbles exposed to pulsed ultrasound. J Med Ultrason (2001) 2005;32:3–11. [PubMed] DOI:10.1007/s10396-005-0031-5
- Kooiman K, Foppen-Harteveld M, van der Steen AF, et al. Sonoporation of endothelial cells by vibrating targeted microbubbles. J Control Release 2011;154:35–41. [PubMed] DOI:10.1016/j.jconrel.2011.04.008
- Wang TY, Choe JW, Pu K, et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J Control Release 2015;203:99–108. [PubMed] DOI:10.1016/j.jconrel.2015.02.018
- Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2012;2:1208–22. [PubMed] DOI:10.7150/thno.4306
- Dixon AJ, Kilroy JP, Dhanaliwala AH, et al. Microbubble-mediated intravascular ultrasound imaging and drug delivery. IEEE Trans Ultrason Ferroelectr Freq Control 2015;62:1674–85. [PubMed] DOI:10.1109/TUFFC.2015.007143
- Bouakaz A, Zeghimi A, Doinikov AA. Sonoporation: Concept and Mechanisms. Adv Exp Med Biol 2016;880:175–89. [PubMed] DOI:10.1007/978-3-319-22536-4_10
- Kotopoulis S, Delalande A, Popa M, et al. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol Imaging Biol 2014;16:53–62. [PubMed] DOI:10.1007/s11307-013-0672-5
- Ren ST, Kang XN, Liao YR, et al. The ultrasound contrast imaging properties of lipid microbubbles loaded with urokinase in dog livers and their thrombolytic effects when combined with low-frequency ultrasound in vitro . J Thromb Thrombolysis 2014;37:303–9. [PubMed] DOI:10.1007/s11239-013-0950-8
- Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J 2003;84:3087–101. [PubMed] DOI:10.1016/S0006-3495(03)70034-4
- Xu WP, Shen E, Bai WK, et al. Enhanced antitumor effects of low-frequency ultrasound and microbubbles in combination with simvastatin by downregulating caveolin-1 in prostatic DU145 cells. Oncol Lett 2014;7:2142–8. [PubMed] DOI:10.3892/ol.2014.2005
- Qin P, Xu L, Han T, et al. Effect of non-acoustic parameters on heterogeneous sonoporation mediated by single-pulse ultrasound and microbubbles. Ultrason Sonochem 2016;31:107–15. [PubMed] DOI:10.1016/j.ultsonch.2015.12.001
- Shapiro G, Wong AW, Bez M, et al. Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation . J Control Release 2016;223:157–64. [PubMed] DOI:10.1016/j.jconrel.2015.12.001
- Dimcevski G, Kotopoulis S, Bjånes T, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 2016;243:172–81. [PubMed] DOI:10.1016/j.jconrel.2016.10.007
- Lin CY, Tseng HC, Shiu HR, et al. Ultrasound sonication with microbubbles disrupts blood vessels and enhances tumor treatments of anticancer nanodrug. Int J Nanomedicine 2012;7:2143–52. [PubMed] DOI:10.2147/IJN.S29514
- Yoon YI, Yoon TJ, Lee HJ. Optimization of ultrasound parameters for microbubble-nanoliposome complex-mediated delivery. Ultrasonography 2015;34:297–303. [PubMed] DOI:10.14366/usg.15009
- Shi D, Guo L, Duan S, et al. Influence of tumor cell lines derived from different tissue on sonoporation efficiency under ultrasound microbubble treatment. Ultrason Sonochem 2017;38:598–603. [PubMed] DOI:10.1016/j.ultsonch.2016.08.022
- Yu H, Xu L. Cell experimental studies on sonoporation: state of the art and remaining problems. J Control Release 2014;174:151–60. [PubMed] DOI:10.1016/j.jconrel.2013.11.010

