Intraperitoneal chemotherapy and its evolving role in management of gastric cancer with peritoneal metastases
Introduction
Peritoneal metastases (PM) of gastric cancer (GC) have been considered a terminal stage of disease (1). The traditional goal of PM of GC has been palliation rather than curative. However, half of the advanced stage GC patients do not respond to chemotherapy with the lowest response rate in GC patients with PM (2). This occurs because PM of GC are penetrated less efficiently than disease at other sites. A poor response to systemic treatment provides the rationale for a local-regional strategy for treatment.
Over the past three decades, a new multimodal treatment called cytoreductive surgery (CRS) with intraperitoneal chemotherapy (IPC) was proposed (3,4). In this review we summarized current knowledge in management of GC with PM.
Rationale of heated IPC in GC with PM
The addition of heat to the IPC treatments was first explored in a pseudomyxoma peritonei patient by Spratt et al. (5). They established both heat and chemotherapy were well tolerated in this patient and had the potential to develop into an effective treatment strategy for patients with the dissemination of cancer to the peritoneal surfaces.
As shown in Table 1, Fujimoto and colleagues performed an early trial in 15 patients with PM developed secondary to advanced GC to test the efficacy of heated intraoperative intraperitoneal chemotherapy (HIPEC) in 1988 (6). They reported acceptable postoperative morbidity and slightly longer mean overall survival (OS) [7.2±4.6 months (mo)].
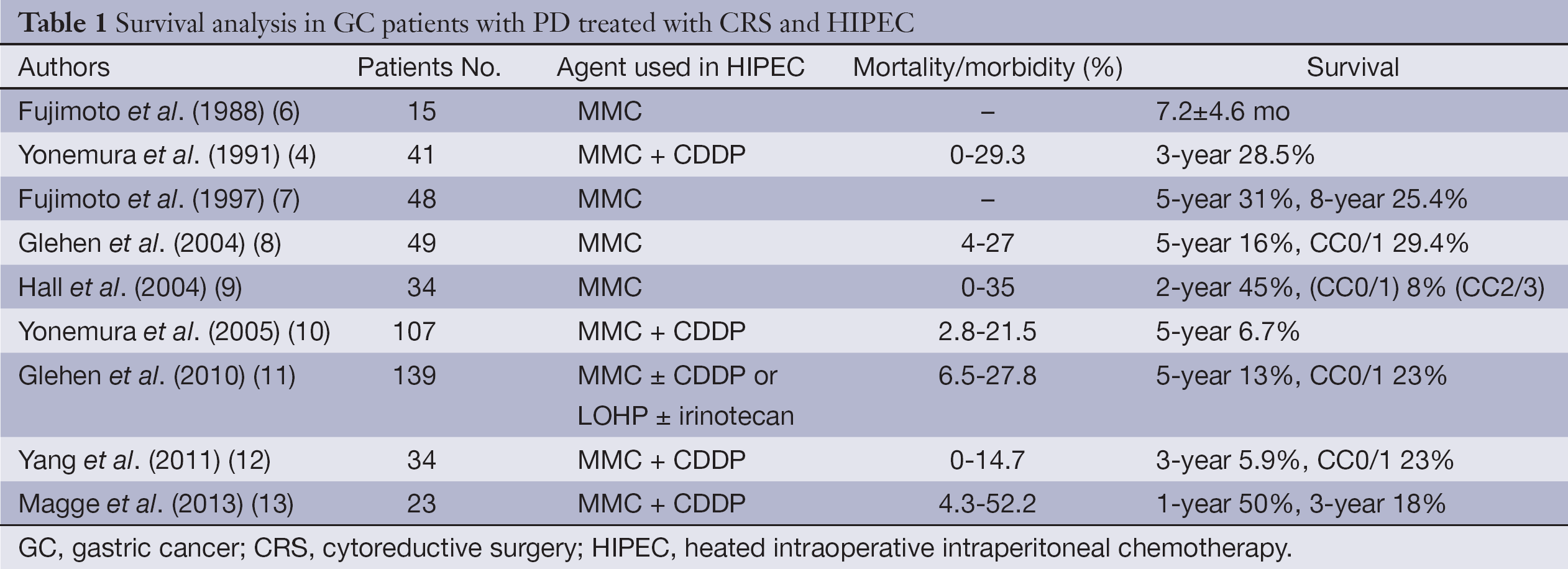
Full table
In 1991, Yonemura and colleagues focused on CRS as an essential component of treatment (4). They reported that CRS plus continuous hyperthermic peritoneal perfusion with mitomycin C (MMC) and cisplatin (CDDP) was performed in 41 GC patients with PM after resection. The overall median survival was 14.6 months and the 3-year survival rate was 28.5%. In 1997, Fujimoto and colleagues performed HIPEC using MMC combined with surgery in 48 GC patients with PM, and reported a 5-year survival of 31%, and 8-year survival 25.4% (7).
Yonemura and colleagues published one of the largest series; when the complete cytoreduction (CC0/1) was achieved 5-year survival rate was in 13% in GC patients with PM compared to 6% in those with incomplete cytoreduction (CC2/3) (10). Completeness of cytoreductive score was described by Jacquet and Sugarbaker (14) and they divided into four in respect of size residual disease left behind following CRS. CC0: no residual disease; CC1: tumor nodules <2.5 mm; CC2: tumor nodules between 2.5 mm to 2.5 cm; and CC3: tumor nodules >2.5 cm. Glehen and colleagues reported the results of 159 patients as a retrospective French multi-institutional study (11). They reported that when complete cytoreduction was achieved 5-year survival rate was increased up to 23% compared to 13% in patients with incomplete cytoreduction.
Effects of bidiractional intraperitoneal and systemic chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in GC patients with PM
Bidirectional intraperitoneal and systemic chemotherapy was developed by Yonemura and colleagues to reduce the tumor burden and to eradicate peritoneal free cancer cells prior to CRS and HIPEC in GC patients with PM (15). This treatment was designed to eradicate dissemination from both peritoneum and subperitoneal blood vessels. Recently, this group published the results of treatment of 194 synchronous and metachronous GC patients with PM (16). Of these 194 patients, 152 (78.3%) patients underwent CRS and HIPEC following bidirectional intraperitoneal and systemic treatment. Treatment-related mortality was 3.9%, and major complications occurred in 23.6% of these patients. The median survival rate was 15.8 months, with 1-, 2-, and 5-year survival rates of 66%, 32% and 10.7%, respectively.
Recently, a meta-analysis on effects of IPC in advanced GC was reported by Coccolini and colleagues (17). They pooled the data from 20 prospective studies involving 2,145 patients. Their conclusion was that IPC benefits GC patients with PM after curative resection. The odds ratio was 0.99 with a 95%confidence interval at 0.71-1.37.
Coccolini and colleagues concluded OS is increased when IPC was added to surgery. OS was not changed with nodal involvement, and mortality rates were not changed with serosal infiltration. IPC was found to reduce the incidence of peritoneal recurrence and distant metastases. They found that morbidity was increased with inraperitoneal chemotherapy. Lymph node involvement was not contraindication for IPC.
Conclusions
These studies suggest that IPC in preoperative and peroperative settings are of benefit in GC with PM. A bidirectional approach combined with CRS and HIPEC was shown to improve OS in GC patients with PM. Further studies will be required for optimize the effects of IPC combined with surgery in these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- Ajani JA, Ota DM, Jessup JM, et al. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer 1991;68:1501-6. [PubMed]
- Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evaluation of treatment strategies for advanced gastric cancer. Semin Surg Oncol 2003;21:233-48. [PubMed]
- Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for treatment of gastric cancer with peritoneal dissemination. World J Surg 1991;15:530-5; discussion 535-6.. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36-41. [PubMed]
- Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997;79:884-91. [PubMed]
- Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004;139:20-6. [PubMed]
- Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454-63. [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370-5. [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2013. [Epub ahead of print]. [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [PubMed]
- Yonemura Y, Bandou E, Kinoshita K, et al. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am 2003;12:635-48. [PubMed]
- Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2013. [Epub ahead of print]. [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [PubMed]
