Overexpression of HOXB9 promotes metastasis and indicates poor prognosis in colon cancer
Introduction
Colon cancer is one of the most prevalent carcinomas throughout the world, and the incidence of colon cancer has shown an upward trend in recent years (1-3). Recently, the 5-year survival rate of colon cancer patients has improved, but the mortality of colon cancer remains high (4,5). The major causes of death are due to local recurrence and distant metastasis. Several genes have been associated with colon cancer metastasis such as vascular endothelial growth factor (VEGF), wingless-type MMTV integration site family, member 5A (WNT5α), and KAI1 COOH-terminal interacting tetraspanin (KITENIN) (6-8). However, the clinical value of these early molecular markers that predict colon cancer metastasis and clinical outcome needs further study. Therefore, there is an urgent need to identify a new biomarker that can be used to predict metastasis and prognosis of colon cancer patients.
Homeobox B9 (HOXB9), a member of the HOX gene family is frequently overexpressed in many tumors (9-14). Emerging evidence links the biological function of HOXB9 to tumor metastasis. The studies have shown that the overexpression of HOXB9 in breast cancer and lung cancer may promote distal metastasis and is associated with clinical outcomes (13,15,16). These biological characteristics suggest that HOXB9 contributes to solid tumor invasion and metastasis. However, the exact roles of HOXB9 in colon cancer have not been clarified.
In this study, we confirmed that HOXB9 was overexpressed in colon cancer. Higher expression of HOXB9 was found to be associated with metastasis and poor survival of colon cancer patients. In addition, a higher expression of HOXB9 was observed in metastases of the lymph node than in non-metastatic lymph nodes. Changes in HOXB9 expression impact the ability of colon cancer cells to invasion and metastases both in vitro and in vivo.
Materials and methods
Human samples
Approval from the Institute Research Ethics Committee was obtained for the use of these clinical materials for research purposes. Colon cancer tissues and adjacent tissues were collected from 128 patients who underwent surgery at the Jiangxi Cancer Hospital from January 2004 to July 2008. One hundred and two samples of the 128 cases had lymph node metastasis. One metastatic lymph node and one non-metastatic lymph node were collected from each patient. All patients were followed up for five years. Follow-up data were collected from the patient records and the files of Jiangxi Cancer Hospital. Patients were followed-up at least every four months from the time of primary resection for the first two years, followed by every six months for five years. But 12.5% of 128 patients were lost to follow-up.
Immunohistochemistry (IHC)
Detection of HOXB9 was performed on 5 mm paraffin sections with the indicated anti-HOXB9 polyclonal antibody (Santa Cruz Biotechnology, CA, USA; dilution 1:250). A peroxidase/3,3'-diaminobenzidine (DAB) secondary antibody (Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer’s instructions. HOXB9 levels were subjectively graded as a function of relative nuclear staining intensity: no or low staining (0-1+) and moderate or high staining (2-3+).
Cell culture and cell line selection
LoVo and SW620 human colon cancer cell lines were purchased from the Shanghai Institute of Cell Biology. LoVo and SW620 cells were maintained in F12K (Sigma, St. Louis, Mo, USA) and L-15 (Gibco, Grand Island, NY, USA) growth media supplemented with 10% fetal bovine serum (FBS; Hyclone, Utah, USA) at 37 °C in a 5% CO2 environment.
Stable knockdown of HOXB9 in SW620 and LoVo cells
pSIREN-RetroQ-TetH carrying the HOXB9 target sequence 5'-CAGACATCCACACACAGTA-3' within the coding sequence of HOXB9 was transfected into the indicated cells in 6-well plates using LipofectamineTM LTX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. And then the shHOXB9 of colon cancer cells was selected by their resistance to hygromycin (800 µg/mL) (Invitrogen, Carlsbad, CA, USA), and one control was included, colon cancer cells that received a negative control vector (shNC).
Plasmid construction and transfection
The human HOXB9 mRNA sequence was obtained from GenBank (Accession number NM_024017.4). The forward primer sequence was 5'-CGCGGATCCTCCATTTCTGGGACGCTTA-3', and the reverse primer sequence was 5'-CCGGAATTCCTTTACTCTTTGCCCTGCTC-3'; a restriction site for BamH I or Xho I was added to the 5' end of the primers separately. The purified PCR product was then subcloned into a TA cloning vector and then into the pcDNATM5/FRT Vector (Invitrogen, Carlsbad, CA, USA). The transfections were performed as described previously (17).
RNA isolation and quantitative real time PCR (qRT-PCR)
Total RNA isolation and qRT-PCR were performed as previously described (13,18). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for HOXB9 detection.
Western blotting analysis
Western blotting was performed according to the detailed procedure previously described (18,19), using rabbit anti-human HOXB9 polyclonal antibody (Santa Cruz Biotechnology, CA, USA). Protein levels were normalized to total β-actin, which was assayed using a mouse monoclonal anti-β-actin antibody (Santa Cruz Biotechnology, CA, USA).
Wound-healing assay
Cells were grown to 80-90% confluence in 60-mm cell culture dishes. A wound was made by scraping a pipette tip across the cell surface after 48 h. The cell movement during wound closure was measured by phase-contrast photography at 37 °C for incubations of 0, 24, and 36 h, and 3 separate experiments were performed
Cell migration and invasion assay
We used 24-well transwell plates with an 8-µm pore size (BD Biosciences, NJ, USA) to determine the effects of HOXB9 on colon cancer cell migration and invasion in vitro. The detailed procedure was performed as previously described (20).
In vivo metastasis assay
SW620 cells (5×106 cells) stably transfected with shHOXB9 or vector were inoculated subcutaneously onto the dorsal surfaces of BALB/c nude male mice (Shanghai SLAC Laboratory Animal Co., Ltd., China). Once xenografts were established, they were excised and minced into 1-mm3 pieces. One of these pieces was then orthotopically implanted at the ileocecal junction of other BALB/c nude mice. The mice were sacrificed 35 d after tumor implantation (21). Animal study was approved by the Ethics Committee for Animal Experiments of the Second Affiliated Hospital of Nanchang University.
Statistical analysis
The results were presented as the 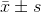 . The correlations between the HOXB9 expression levels and clinicopathological variables were analyzed using Pearson’s Chi-squared test. The survival curve of patients was calculated by the Kaplan-Meier method. Statistical differences between groups were evaluated by one-way analysis of variance (ANOVA). A Fisher’s least significant difference (LSD test) was used for multiple comparisons. Test results were considered significant at P<0.05.
. The correlations between the HOXB9 expression levels and clinicopathological variables were analyzed using Pearson’s Chi-squared test. The survival curve of patients was calculated by the Kaplan-Meier method. Statistical differences between groups were evaluated by one-way analysis of variance (ANOVA). A Fisher’s least significant difference (LSD test) was used for multiple comparisons. Test results were considered significant at P<0.05.
Results
HOXB9 expression is frequently upregulated in colon cancer
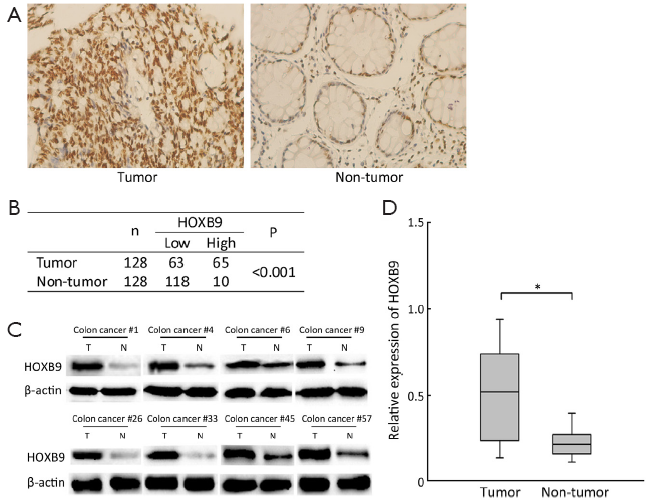
The protein expression levels of HOXB9 in 128 pairs of colon cancer and their corresponding non-tumorous colon were detected via IHC and Western blotting. The IHC results showed that high level of HOXB9 expression was observed in 50.8% (65/128) of the cancer tissues. While only 7.8% (10/128) of the cancer-adjacent normal tissues showed high expression of HOXB9 (Figure 1A,B). The expression ratio obtained via Western blotting was consistent with the results of IHC (Figure 1C,D). These results indicated that there is overexpression of HOXB9 in colon cancer tissues compared to normal tissues.
Overexpression of HOXB9 is associated with metastasis and prognosis in colon cancer
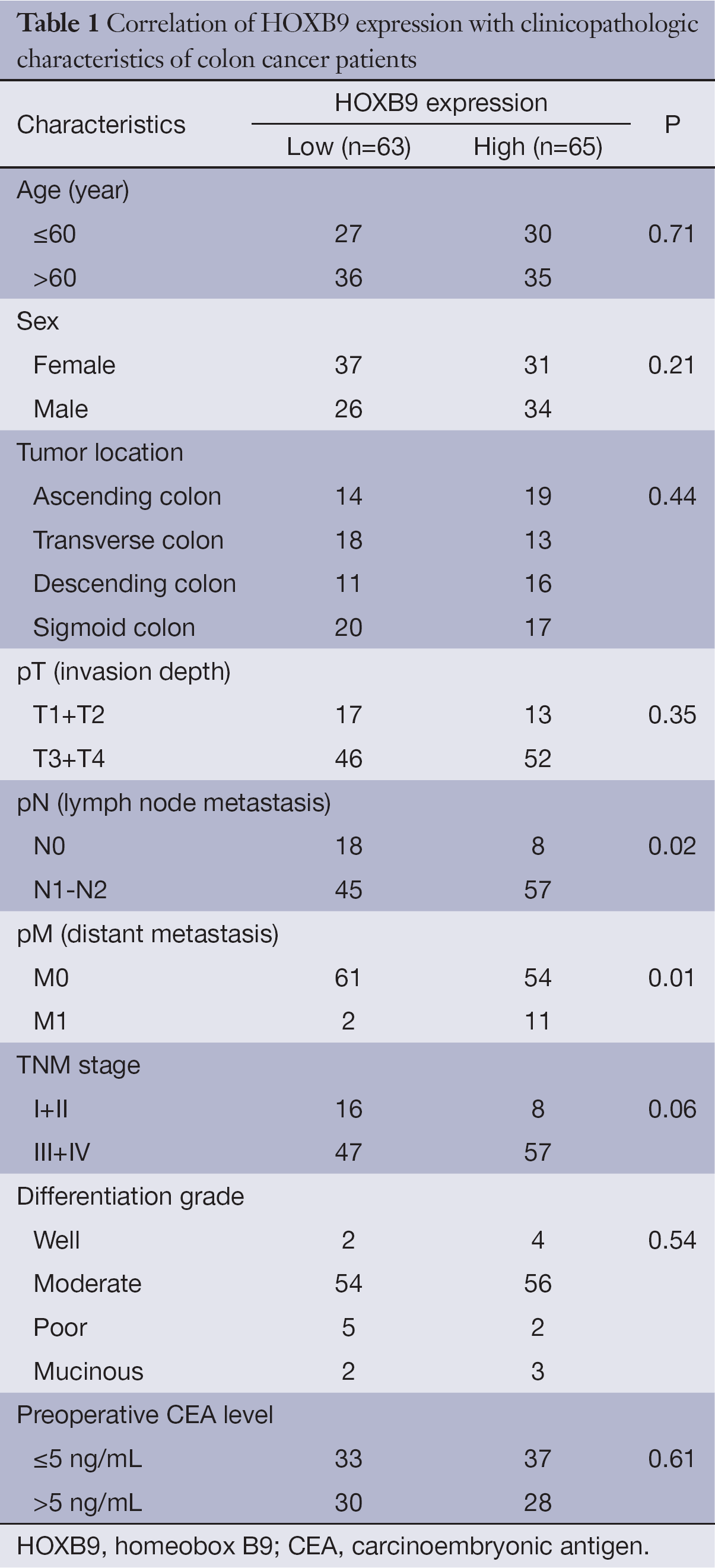
The correlations between the intensity of HOXB9 expression and pathologic variables of the patients were analyzed. There was a significantly positive correlation between high HOXB9 expression and the number of lymph node metastases and distant metastasis (Table 1). Tumors with higher expression levels of HOXB9 were correlated with a lower 5-year survival rate (n=128, P=0.01) (Figure 2A).
Full table
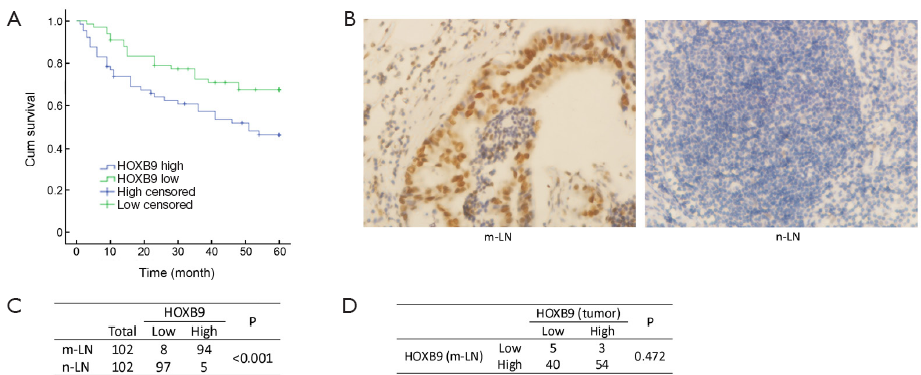
The expression of HOXB9 was also assessed in 102 metastatic lymph nodes and 102 non-metastatic lymph nodes by IHC. The results revealed that 92.2% (94/102) of the metastatic lymph nodes showed high levels of HOXB9 expression. Only 4.90% (5/102) of the non-metastatic lymph nodes exhibited high expression of HOXB9 (Figure 2B,C), demonstrating that the overexpression of HOXB9 was observed in metastatic lymph nodes (P<0.01).
Our study further analyzed the expression of HOXB9 in the 102 metastatic lymph nodes corresponding to colon cancer tissue samples. The results showed that for the 57 colon cancer tissues with high expression of HOXB9, 94.7% (54/57) of the corresponding metastatic lymph nodes have high levels of HOXB9 expression, and only 5.26% (3/57) of these tumor samples have low expression levels of HOXB9. For the 45 colon cancer tissues with low HOXB9 expression, 88.9% (40/45) of the corresponding metastatic lymph nodes also have high expression levels of HOXB9, and 11.1% (5/45) have low expression of HOXB9 (Figure 2D). These results suggested that, regardless of the HOXB9 expression levels in tumor tissues, the proportion of corresponding metastatic lymph nodes with HOXB9 overexpression is very high.
Changes in HOXB9 expression in colon cancer cells affect cell migration and invasion in vitro
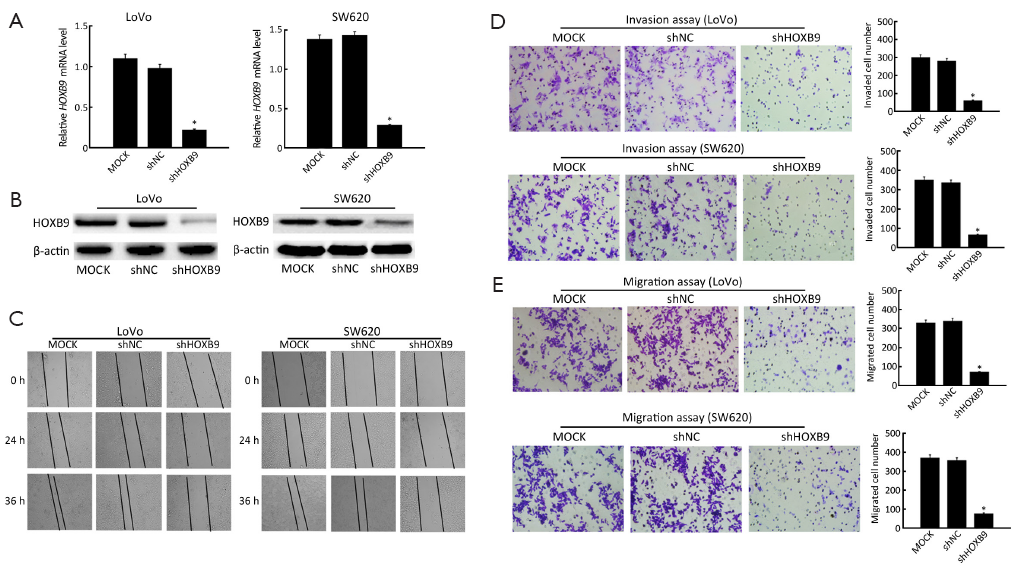
We next investigated the effects of HOXB9 on colon cancer cell migration and invasion. First, LoVo and SW620 cells were stably transfected with the shRNA-HOXB9 construct. The experiment included MOCK, shNC and shHOXB9 groups. The qRT-PCR and Western blotting results showed that HOXB9 mRNA and protein expressions were significantly down-regulated in the shHOXB9 group (Figure 3A,B). At the same time, we found that the artificial wound became significantly wider in the shHOXB9 group after 24 and 36 h (Figure 3C). The transwell assay also showed that the knockdown of HOXB9 could significantly decrease cell migration and invasion (Figure 3D,E).
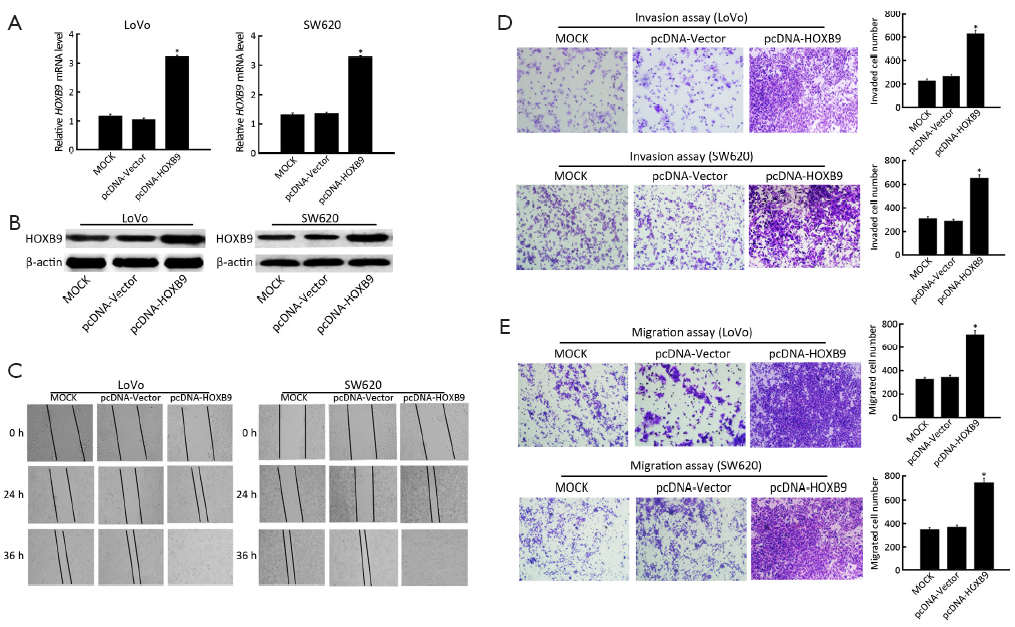
To further reveal the role of HOXB9 in the regulation of colon cancer cell migration and invasion, the pcDNA-HOXB9 plasmid was transfected into LoVo and SW620 cells. The experiment included MOCK, pcDNA-Vector and pcDNA-HOXB9 groups. The results showed that the expression of HOXB9 was significantly up-regulated in the pcDNA-HOXB9 group (Figure 4A,B). The wound-healing and transwell assay results indicated that increased HOXB9 expression in LoVo and SW620 cells could significantly enhance cell migration and invasive ability (Figure 4C-E).
Stable knockdown of HOXB9 in colon cancer cell line suppresses metastasis in vivo
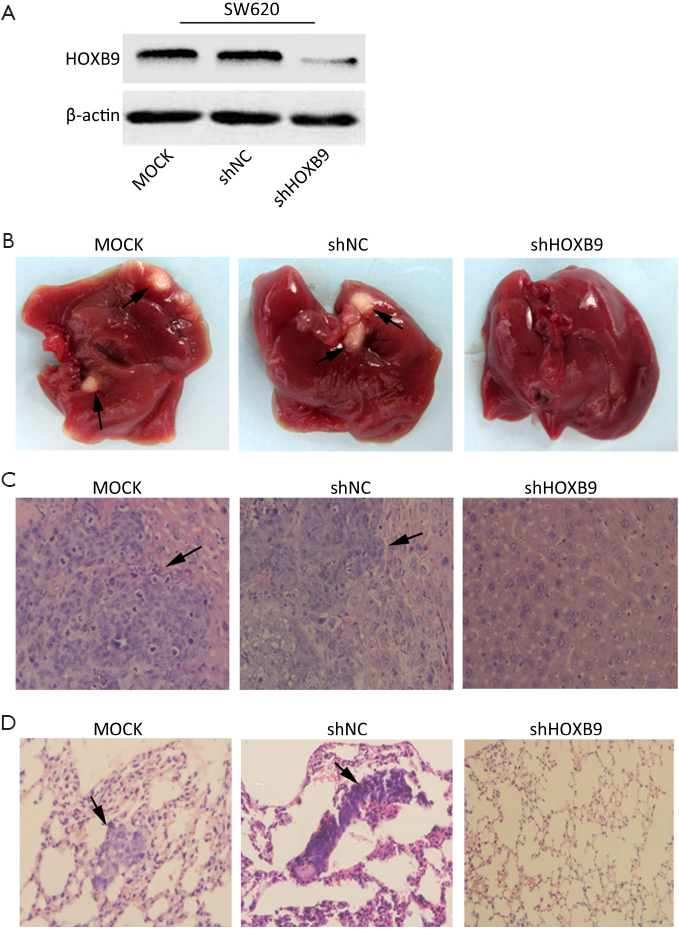
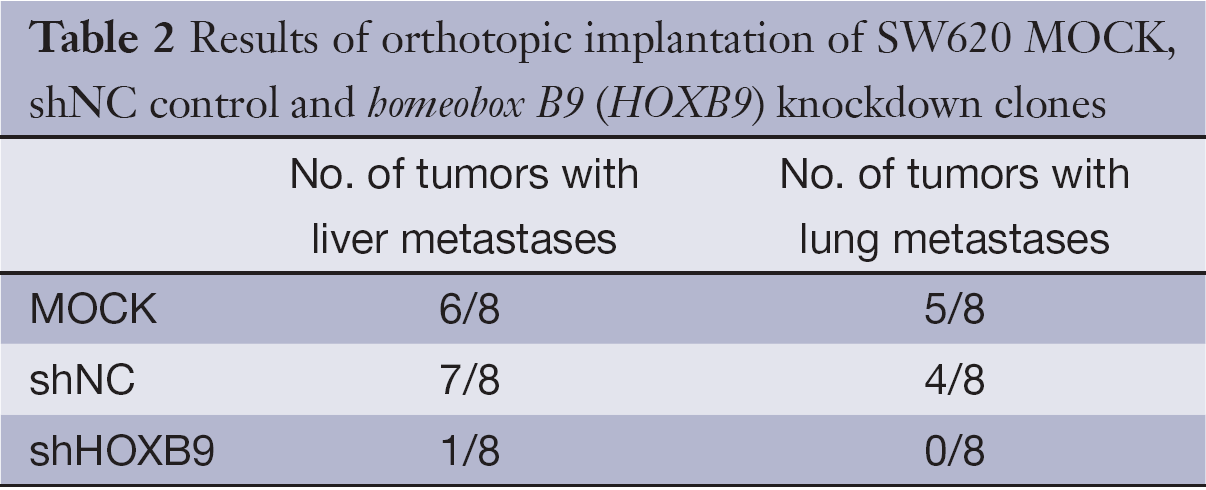
We next tested whether HOXB9 knockdown inhibits tumor metastasis in vivo. The SW620 cells with stable knockdown of HOXB9 were used to establish an orthotopic tumor model in nude mice (Figure 5A). The experiment included MOCK, shNC and shHOXB9 groups. Gross specimens and histological examination indicated that the number of metastatic nodules of the liver and lung was significantly decreased in shHOXB9 group (Figure 5B-D, Table 2). Together these results indicated that the knockdown of HOXB9 can suppress distant metastasis of colon cancer cells in vivo.
Full table
Discussion
In the present study, we report that HOXB9 was overexpressed in human colon cancer tissues. The overexpression of HOXB9 is significantly associated with aggressive phenotypes and adverse prognosis of colon cancer patients. The expression of HOXB9 in metastatic lymph nodes was significantly higher than that of non-metastatic lymph nodes. In addition, down-regulating the expression of HOXB9 inhibited the migration and invasion of colon cancer cells, and the exogenous expression of HOXB9 in colon cancer cells enhanced cell migration and invasiveness. We lastly also demonstrated that the knockdown of HOXB9 can decrease colon cancer metastasis to the liver and lungs in an orthotopic model of colon cancer.
HOXB9 belongs to the homeobox transcription factor gene family, which is critical for embryonic segmentation and limb patterning (22-25). Accumulative evidence suggests that HOXB9 is overexpressed in many solid tumors, including lung cancer, Hodgkin’s lymphoma and breast cancer (9-11). Elevated HOXB9 expression in breast carcinoma is correlated with high tumor grade, the number of pathologic nodal metastases and poor survival (13,15). In this study, we conformed that HOXB9 was overexpressed in colon cancer tissue. The overexpression of HOXB9 was also associated with number of lymph node metastasis, distant metastasis and poor 5-year survival performance of colon cancer patients. These results indicated that HOXB9 may be a potential metastasis and prognosis biomarker for colon cancer patients.
Lymph node and distant metastasis is the major cause of poor survival in patients with colon cancer (26-28). We therefore further assessed the expression of HOXB9 in metastatic and non-metastatic lymph nodes of colon cancer. The results indicated that that higher expression of HOXB9 was observed in lymph node metastases than in non-metastatic lymph nodes. In addition, we also analyzed the expression of HOXB9 in 102 metastatic lymph nodes and their corresponding colon cancer tissue samples. The results suggested that 94.7% (54/57) of metastatic lymph nodes corresponding to high-HOXB9 tumors have high expression of HOXB9. Interestingly, we also found that 88.9% (40/45) of metastatic lymph nodes corresponding to low-HOXB9 colon cancer tissues also have high expression of HOXB9. This result indicates that HOXB9 may play an important role in the lymph node metastasis of colon cancer, but the specific mechanism of this process needs further study. Because HOXB9 was overexpressed in the metastatic lymph nodes of colon cancer patients but largely absent from the non-metastatic lymph nodes, surgeons could potentially design fluorescently labeled probes specific for HOXB9 to distinguish metastatic lymph nodes and normal lymph nodes, guide intraoperative lymph node dissection, and improve the postoperative detection rate for lymph node micrometastases in colon cancer.
The exact molecular mechanism by which HOXB9 regulates invasion and metastasis of malignant tumors are still not completely clear. The previous findings suggested that ectopic expression of HOXB9 could promote angiogenesis of breast cancer and distal metastasis of lung cancer (13,16,29). Our results showed that knockdown of HOXB9 can inhibit migration and invasion of colon cancer cells. In contrast, increased the expression of HOXB9 could enhance cell migration and invasion. In addition, in vivo xenograft tumor metastasis assays demonstrated that the stable knockdown of HOXB9 in colon cell lines could also suppress tumor metastasis to lung and liver. These results indicate that HOXB9 plays an important role in the invasion and metastasis of colon cancer.
In conclusion, our study demonstrated that the elevated expression of HOXB9 correlates with lymph node metastasis, distant metastasis and poor survival in colon cancer patients. This study also provided evidence that the expression level of HOXB9 is critical for colon cancer cell invasion and metastasis in vitro and in vivo. We speculate that HOXB9 may be a potential biomarker for poor prognosis and complete lymph node dissection of colon cancer patients.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No.81060196), the Scientific Research Project Foundation of Jiangxi Provincial Education Department (No. GJJ11333), and Jiangxi Provincial Major Disciplines of Academic and Technical Leaders Project (No. 20113BCB22004).
Disclosure: The authors declare no conflict of interest.
References
- Naishadham D, Lansdorp-Vogelaar I, Siegel R, et al. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:1296-302. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol 2013;19:8515-26. [PubMed]
- Lai YL, Lin JK, Liang WY, et al. Surgical resection combined with chemotherapy can help achieve better outcomes in patients with primary colonic lymphoma. J Surg Oncol 2011;104:265-8. [PubMed]
- Mann O, Strate T, Schneider C, et al. Surgery for advanced and metastatic pancreatic cancer--current state and perspectives. Anticancer Res 2006;26:681-6. [PubMed]
- Martins SF, Garcia EA, Luz MA, et al. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics 2013;10:55-67. [PubMed]
- Bakker ER, Das AM, Helvensteijn W, et al. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis 2013;34:2629-38. [PubMed]
- Lee JK, Bae JA, Sun EG, et al. KITENIN increases invasion and migration of mouse squamous cancer cells and promotes pulmonary metastasis in a mouse squamous tumor model. FEBS Lett 2009;583:711-7. [PubMed]
- Calvo R, West J, Franklin W, et al. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci U S A 2000;97:12776-81. [PubMed]
- Nagel S, Burek C, Venturini L, et al. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 2007;109:3015-23. [PubMed]
- Shrestha B, Ansari KI, Bhan A, et al. Homeodomain-containing protein HOXB9 regulates expression of growth and angiogenic factors, facilitates tumor growth in vitro and is overexpressed in breast cancer tissue. FEBS J 2012;279:3715-26. [PubMed]
- de Pinieux G, Legrier ME, Poirson-Bichat F, et al. Clinical and experimental progression of a new model of human prostate cancer and therapeutic approach. Am J Pathol 2001;159:753-64. [PubMed]
- Hayashida T, Takahashi F, Chiba N, et al. HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci U S A 2010;107:1100-5. [PubMed]
- Yamagishi T, Hirose S, Kondo T. Secondary DNA structure formation for Hoxb9 promoter and identification of its specific binding protein. Nucleic Acids Res 2008;36:1965-75. [PubMed]
- Seki H, Hayashida T, Jinno H, et al. HOXB9 expression promoting tumor cell proliferation and angiogenesis is associated with clinical outcomes in breast cancer patients. Ann Surg Oncol 2012;19:1831-40. [PubMed]
- Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 2009;138:51-62. [PubMed]
- Liu T, Yu X, Li G, et al. Rock2 regulates Cdc25A through ubiquitin proteasome system in hepatocellular carcinoma cells. Exp Cell Res 2012;318:1994-2003. [PubMed]
- Peng X, Shao J, Shen Y, et al. FAT10 protects cardiac myocytes against apoptosis. J Mol Cell Cardiol 2013;59:1-10. [PubMed]
- Yu X, Liu X, Liu T, et al. Identification of a novel binding protein of FAT10: eukaryotic translation elongation factor 1A1. Dig Dis Sci 2012;57:2347-54. [PubMed]
- Wong CC, Wong CM, Tung EK, et al. Rho-kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology 2009;49:1583-94. [PubMed]
- Wang J, Rajput A, Kan JL, et al. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J Biol Chem 2009;284:10912-22. [PubMed]
- Ansari KI, Shrestha B, Hussain I, et al. Histone methylases MLL1 and MLL3 coordinate with estrogen receptors in estrogen-mediated HOXB9 expression. Biochemistry 2011;50:3517-27. [PubMed]
- Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci U S A 1999;96:541-6. [PubMed]
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2002;2:777-85. [PubMed]
- Xu B, Wellik DM. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc Natl Acad Sci U S A 2011;108:4888-91. [PubMed]
- Wanebo HJ, LeGolvan M, Paty PB, et al. Meeting the biologic challenge of colorectal metastases. Clin Exp Metastasis 2012;29:821-39. [PubMed]
- Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 2010;17:2847-55. [PubMed]
- Nesbakken A, Gaard M. Surgical treatment of colon cancer. Tidsskr Nor Laegeforen 2007;127:2942-5. [PubMed]
- Chiba N, Comaills V, Shiotani B, et al. Homeobox B9 induces epithelial-to-mesenchymal transition-associated radioresistance by accelerating DNA damage responses. Proc Natl Acad Sci U S A 2012;109:2760-5. [PubMed]
