Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone
Introduction
Hepatocellular carcinoma (HCC, also called malignant hepatoma) is one of the most common and severe malignancies in China. HCC ranks fifth for men and eighth for women, and accounts for >660,000 new cases annually worldwide (1-3). Most cases of HCC are secondary to either a viral hepatitis infection (hepatitis B or C) or cirrhosis (alcoholism being the most common cause of hepatic cirrhosis). Since radiation and chemotherapy are not effective for HCC, surgical resection provides the best potentially curative option. Unfortunately, due to poor baseline liver function, over tumor burden, or hepatic vessel invasion of HCC patients, it is barely possible to perform surgical resection. During the past decade, locoregional treatments have been developed rapidly, such as transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), and microwave ablation (MWA). RFA or MWA as a thermal in situ destruction technique has been proved to be a safe and effective treatment. It has been accepted as one of the best treatment options for small HCC (4,5). TACE as a palliative therapy has become one of the most widely performed treatments for unresectable HCC (6,7). They all are minimally invasive options that may individually or in combination achieve the pertinent balance in successful tumor eradication and maximal preservation of liver function.
TACE slows tumor progression and improves survival by combining the effect of targeted chemotherapy with ischemic necrosis by arterial embolization. However, the complete necrosis rate of tumors after TACE only reaches 10-20%, and the 1-, 3- and 5-year overall survival rates range from 49.0-71.9%, 23.0-62.5% and 9-17% in most studies (8-13). TACE is the most commonly used therapy for intermediate-stage HCC (5,6). RFA or MWA has emerged as an accepted therapy for small HCC (14). However, it is difficult for RFA or MWA to achieve complete ablation in the treatment of relatively large HCC.
Therefore, either TACE or RFA/MWA has its own limitations, in particular, neither can result in adequate control of medium or large HCC (5,15-17). In recent years, the combination of interventional therapies has been widely performed for treatment of HCC. TACE decreases blood flow to the tumor, making subsequent RFA or MWA more effective, as there is less heat loss by convection (18). Several studies have demonstrated the synergistic cytotoxic effects of TACE with RFA for HCC (19). Whether RFA or MWA combined with TACE or RFA monotherapy is the better treatment choice for HCC has long been debated. This is such a study coming from a single tertiary referral center conducted on patients with HCC ≤7 cm.
Materials and methods
Patients
The study was a prospective, randomized, controlled trial conducted at the Department of Hepatobiliary Surgery, the Second Affiliated Hospital of Southeast University, Nanjing, China. From June 2008 to June 2010, patients with HCC who met the entry criteria and agreed to participate were included in the study. HCC was diagnosed, staged, and treated according to a previously reported schedule (20).
The eligibility criteria were as follows: (I) age 18 to 70 years; (II) patient has signed informed consent; (III) a solitary HCC ≤7.0 cm in diameter, or multiple HCC ≤3 lesions, each ≤3.0 cm in diameter; (IV) no evidence of extrahepatic disease; (V) lesions being visible on ultrasound (US) and with an acceptable/safe path between the lesion and the skin as shown on US; (VI) no history of encephalopathy, ascites refractory to diuretics or variceal bleeding; (VII) no previous treatment of HCC except liver resection; (VIII) surgical operation failure and postoperative recurrence; and (VIIII) Child-Pugh class A or B cirrhosis.
The exclusion criteria were: (I) patient compliance is poor; (II) severe coagulation disorders [prothrombin activity <40% or a platelet count (PLT) of <40,000/µL]; (III) the blood supply of tumor lesions is absolutely poor or arterial-venous shunt so that TACE cannot be performed; (VI) contraindications to carboplatin, epirubicin, mitomycin, or lipiodol; or (V) history of cardiac disease.
Patients who met these criteria and gave written informed consent entered the study, which was approved by the Ethic Committee of the hospital. It conformed to the standards of the Declaration of Helsinki.
Patients were randomly assigned into the TACE-RFA or TACE-MWA group and the RFA-alone or MWA-alone group. Randomization was done using a computer-generated algorithm with numbers by a nurse who was not part of this research team. Double-blind and double-dummy techniques were discarded because the nature of the treatment and their possible adverse effects. The interval between randomization and the treatment was less than two weeks. Treatment was discontinued if any exclusion criteria developed or at the patient’s request.
Treatment procedure and follow up
All TACE and RFA/MWA were performed by the same team of doctors. For combined RFA or MWA with TACE (combined treatment group), first TACE was performed according to the following protocol: arterial portography was performed for diagnosis of the patency of the portal vein; 25-30 mL of 65% iopamidol was injected via a 4.0-F preshaped catheter (Selecon-PA Catheter); all patients underwent a distal super-selective catheterization of the hepatic arteries using a coaxial technique and microcatheters (2.9 F; Terumo Corporation, Tokyo, Japan). Then the same three chemotherapeutic agents were administered, carboplatin 300 mg (Bristol-Myers Squibb, New York, NY, USA), epirubicin hydrochloride 50 mg (Pharmorubicin; Pfizer, Wuxi, China), and mitomycin 8 mg (Zhejiang Hisun Pharmaceutical, Taizhou, China).
RFA or MWA was performed a week after TACE by using a commercially available system (RF 2000; Radio-Therapeutics, Mountain View, CA, USA). RFA was applied until either there was a marked increase in impedance or 15 min had elapsed. If the marked increases in impedance were not obtained, the second treatment was carried out within 15 min. No more than three applications of RFA or MWA were given in a treatment session. Tumors less than 3 cm in their greatest diameter can be ablated with single ablation. Tumors more than 3 cm in their greatest diameter should be treated with multiple overlapping ablations (21). In the TACE-RFA group, because gelatin sponge remains in the tumor for 2 weeks after chemoembolization, 24 RFA or MWA treatments followed TACE within 2 weeks (median, 7 d; range, 3 to 14 d).
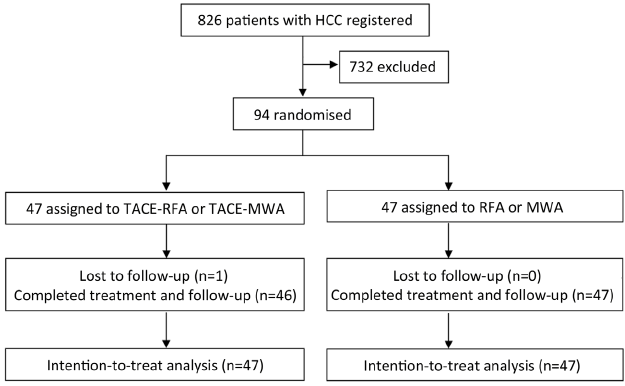
Treatment response was assessed by contrast-enhanced spiral computed tomography (CT). Thereafter, the patients were followed up once every 3 months for the first 2 years, once every 6 months from 2 to 5 years after treatment and then to once every 12 months after 5 years. All CT scans were reviewed by two radiologists who were unaware of patient clinical data or treatment assignment. At each follow-up visit, US and blood tests including serum liver function tests and α-fetoprotein (AFP) were carried out. No patient received antiviral treatment during the trial. Chest radiography, ultrasonography, serum biochemistry, and clinical examination were performed once every 6 months. The treatment course and follow-up are shown in Figure 1.
Statistical analysis
The statistical analyses were performed using the SPSS 13.0 software (SPSS, Chicago, IL, USA). Differences in 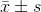 were analyzed by student’s paired t-test. Differences in the median were analyzed by χ2 test. The survival curves according to the Kaplan-Meier method were compared by the Mantel-Cox test, stratified by tumor size and tumor number. Multivariant Cox’s proportional hazard regression model was used to analyze the relative prognostic significance of the variables in predicting overall survival rates. P<0.05 was considered statistically significant.
were analyzed by student’s paired t-test. Differences in the median were analyzed by χ2 test. The survival curves according to the Kaplan-Meier method were compared by the Mantel-Cox test, stratified by tumor size and tumor number. Multivariant Cox’s proportional hazard regression model was used to analyze the relative prognostic significance of the variables in predicting overall survival rates. P<0.05 was considered statistically significant.
Results
Enrollment
From June 2008 to June 2010, 94 (11.4%) of 826 patients diagnosed as HCC met the entry criteria and agreed to take part in the study, 622 patients did not participate, and 110 patients did not meet the inclusion criteria of the study.
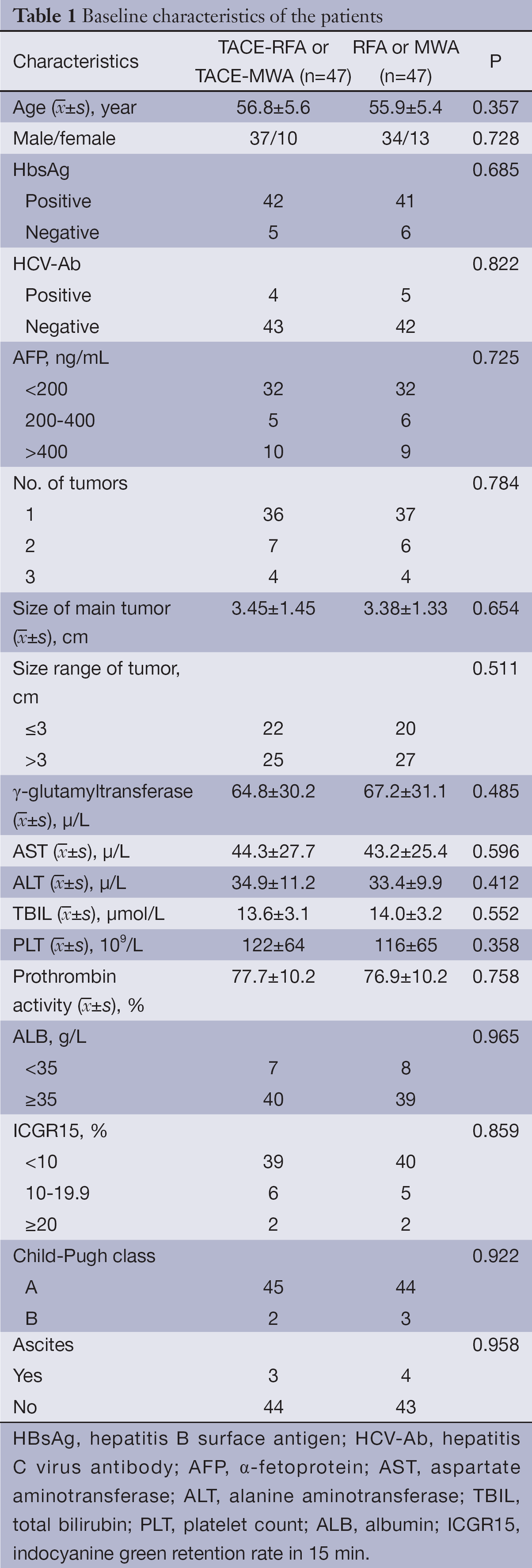
Of 94 patients who were eligible and consented to be randomly assigned, 47 patients were assigned to the RFA-alone or MWA-alone group, and 47 to the TACE-RFA group or TACE-MWA group (Figure 1). Table 1 lists the baseline characteristics of the patients. There were no significant differences among the two groups for any of the variables.
Full table
Survivals
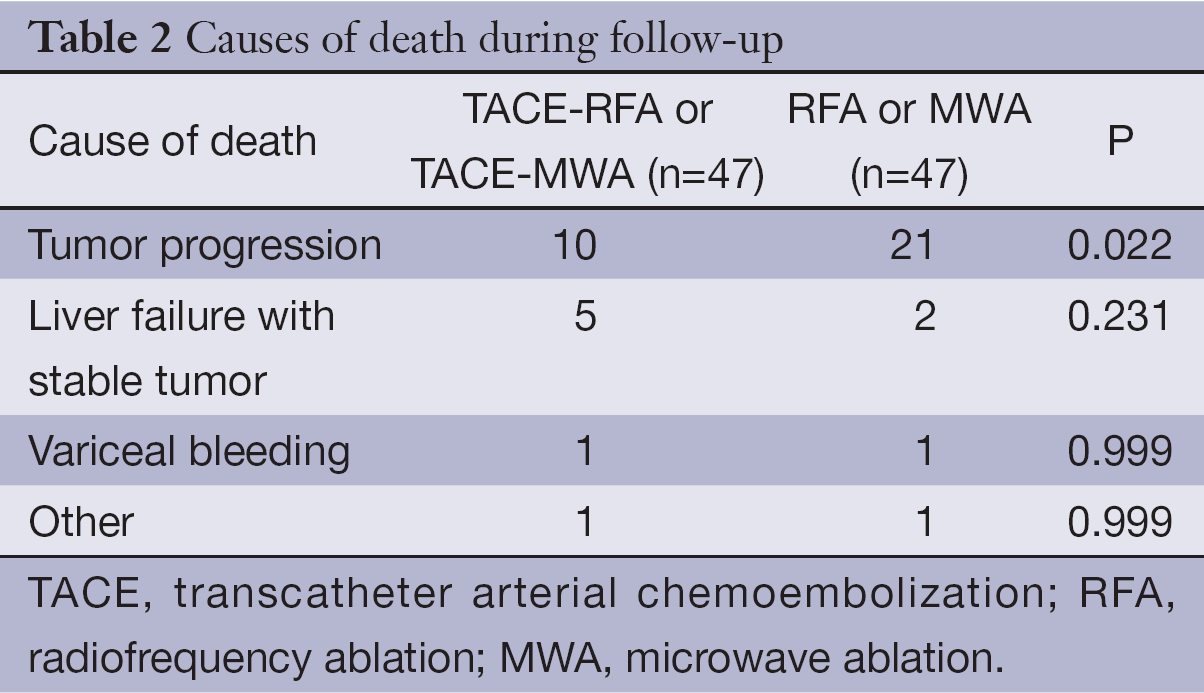
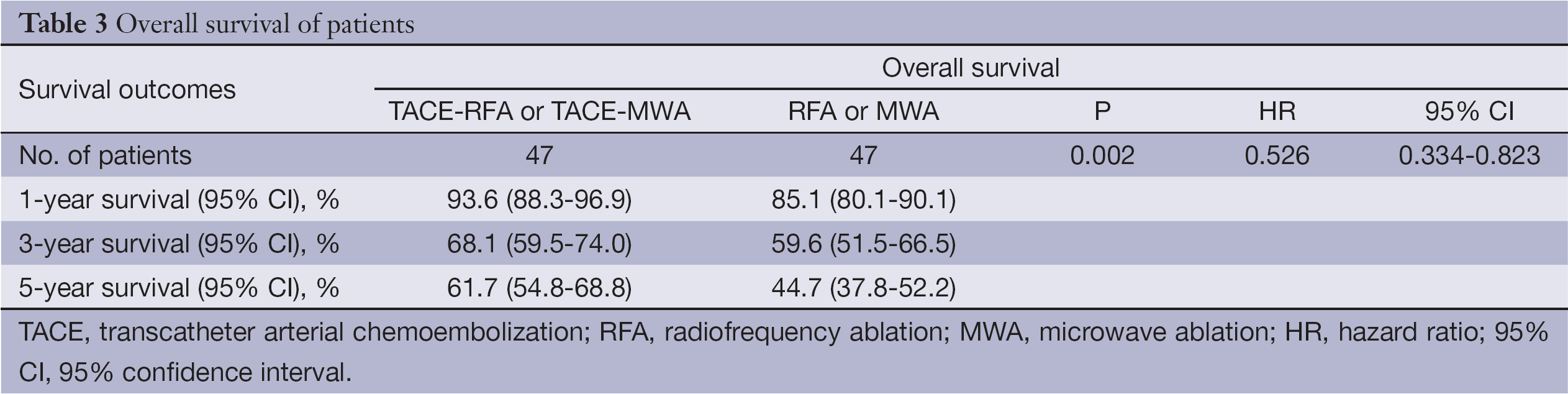
Until the time of censor, 17 patients in the TACE-RFA and TACE-MWA group had died. The median follow-up time of the patients who were still alive for the TACE-RFA or TACE-MWA group was 47.5±11.3 months (range, 29 to 62 months) (Table 2). The 1-, 3- and 5-year overall survival for the TACE-RFA or TACE-MWA group was 93.6%, 68.1% and 61.7%, respectively (Table 3). Twenty-five patients in the RFA or MWA group had died. The median follow-up time of the patients who were still alive for the RFA or MWA group was 47.0±12.9 months (range, 28 to 62 months) (Table 2). The 1-, 3-, and 5-year overall survival for the RFA or MWA group were 85.1%, 59.6% and 44.7%, respectively (Table 3).
Full table
Full table
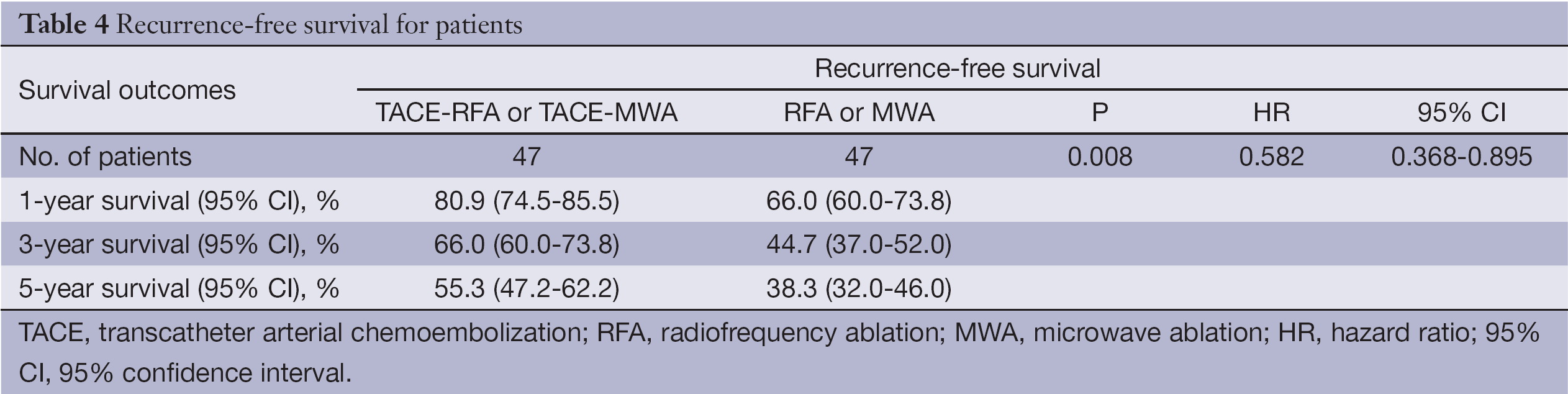
Using the Cox proportional hazards regression model, analysis was performed for each group. The patients in the TACE-RFA or TACE-MWA group had better overall survival than the RFA or MWA group [hazard ratio (HR), 0.526; 95% confidence interval (95% CI), 0.334-0.823; P=0.002] (Table 3), and showed better recurrence-free survival than the RFA or MWA group (HR, 0.582; 95% CI, 0.368-0.895; P=0.008) (Table 4).
Full table
Complications
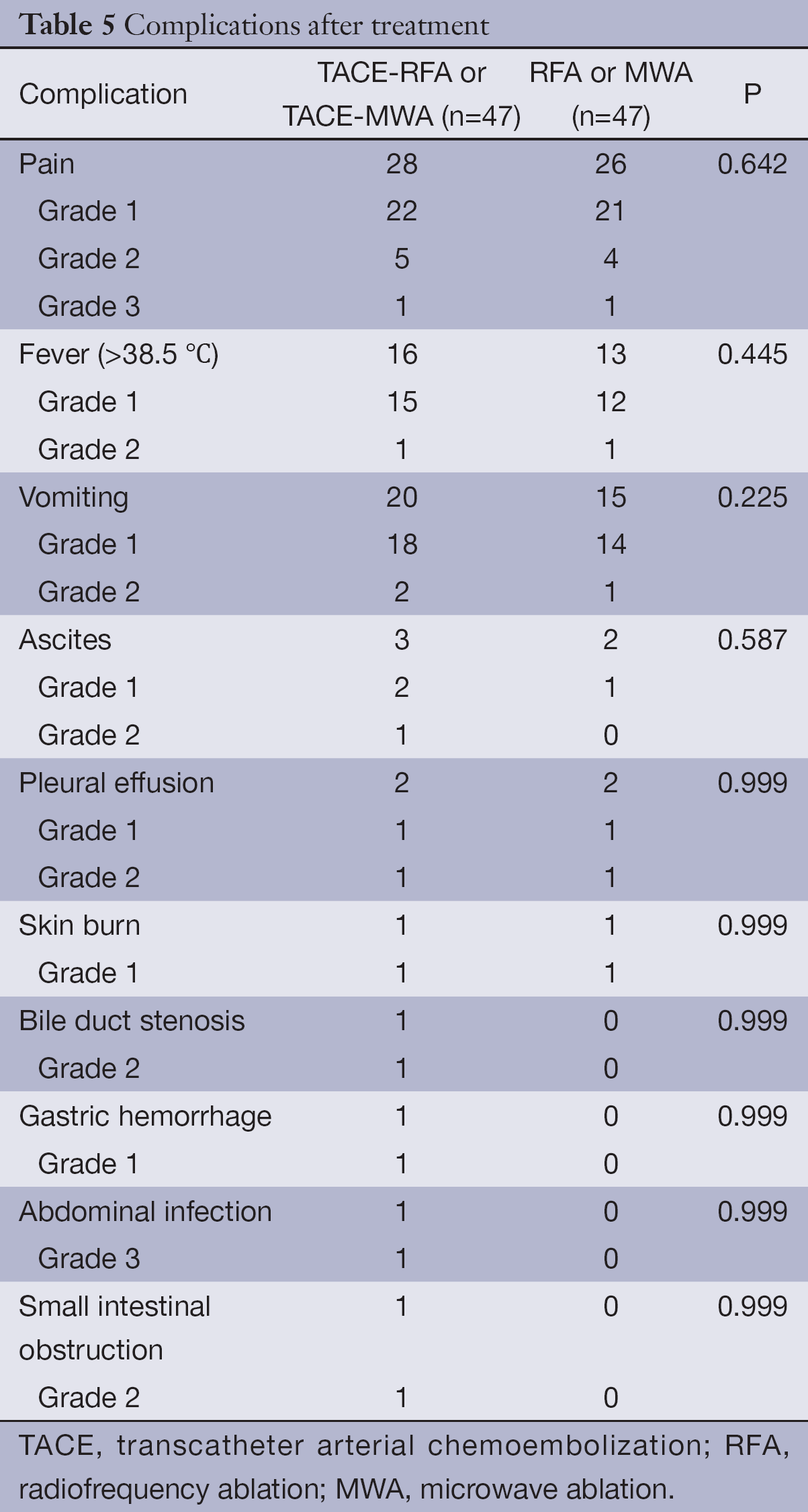
There were no treatment-related deaths, and no serious complications were observed after TACE and RFA/MWA treatment. Common complications of TACE and RFA/MWA were fever, pain, vomiting, ascites, pleural effusion, and skin burn. Other complications included bile duct stenosis and gastric hemorrhage in the TACE-RFA or TACE-MWA group, and abdominal infection and small intestinal obstruction in the RFA or MWA group (Table 5).
Full table
Discussion
RFA is usually performed to treat small (≤3 cm) HCC nodules (22). However, the limited coagulative necrosis induced by RFA or MWA alone and the occasionally irregular burn shape caused by the heat-sink effect of large vessels in the proximity of the ablated area prevented the widespread use of RFA for the treatment of intermediate- to large-sized hepatic tumors. To obtain a large coagulation area, various techniques have been advocated (22-27). We conducted a randomized controlled trial to evaluate the advantages of combining RFA or MWA with TACE for treating patients with HCC. This study showed that TACE-RFA or TACE-MWA gives better efficacy than RFA or MWA alone for HCC ≤7 cm. TACE before RFA/MWA is beneficial because it enables better ablation than that achieved with RFA or MWA alone and possibly facilitates the effective treatment of patients with HCC. TACE can block hepatic arterial blood flow and attenuate the cooling effect of tumoral arterial blood flow (8,19). Furthermore, iodized oil and gelatin sponge particles used in TACE can increase RFA or MWA-induced coagulation necrosis by going through multiple arterioportal communications and effects are added in terms of ablation time, maximum output, and portal angiography by TACE with iodized oil. Several studies also revealed that recurrent tumors commonly occurred in the liver remnant at a segment distant from the resection margin or at multiple liver segments (28,29). Therefore, an enlarged ablation zone improves the chance of total ablation of micrometastasis, reduces the risk of tumor recurrence for patients with HCC, enhances the effect of hyperthermia and controls microlesions (30).
In conclusion, RFA or MWA combined with TACE in the treatment of HCC ≤7 cm was superior to RFA or MWA alone in improving survival by reducing arterial and portal blood flow due to TACE with iodized oil before RFA.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferenci P, Fried M, Labrecque D, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol 2010;44:239-45. [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-6. [PubMed]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201-9. [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [PubMed]
- El Awady MK, Bader El Din NG, Tabll A, et al. IL28B polymorphism and cytomegalovirus predict response to treatment in Egyptian HCV type 4 patients. World J Gastroenterol 2013;19:290-8. [PubMed]
- Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med 1999;20:47-53. [PubMed]
- Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127:S179-88. [PubMed]
- Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol 2012;39:503-9. [PubMed]
- Marelli L, Shusang V, Buscombe JR, et al. Transarterial injection of (131)I-lipiodol, compared with chemoembolization, in the treatment of unresectable hepatocellular cancer. J Nucl Med 2009;50:871-7. [PubMed]
- Kim KM, Kim JH, Park IS, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009;24:806-14. [PubMed]
- Farinati F, Giacomin A, Vanin V, et al. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol 2012;57:221-2. [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 1999;19:329-38. [PubMed]
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rate after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9. [PubMed]
- N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475-83. [PubMed]
- Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: A long-term cohort study. Hepatology 2011;53:136-47. [PubMed]
- Sturm JW, Keese M. Multimodal treatment of hepatocellular carcinoma (HCC). Onkologie 2004;27:294-303. [PubMed]
- Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000;217:119-26. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [PubMed]
- Chen MH, Yang W, Yan K, et al. Large liver tumors: Protocol for radiofrequency ablation and its clinical application in 110 patients-Mathematic model, overlapping mode, and electrode placement process. Radiology 2004;232:260-71. [PubMed]
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 2009;252:905-13. [PubMed]
- Peng ZW, Chen MS, Liang HH, et al. A case control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur J Surg Oncol 2010;36:257-63. [PubMed]
- Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol 2006;16:661-9. [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: Transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 2011;18:1624-9. [PubMed]
- Zhang YJ, Liang HH, Chen MS, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: A prospective randomized trial. Radiology 2007;244:599-607. [PubMed]
- Giorgio A, Tarantino L, de Stefano G, et al. Percutaneous sonographically guided saline-enhanced radiofrequency ablation of hepatocellular carcinoma. AJR Am J Roentgenol 2003;181:479-84. [PubMed]
- Yoshida Y, Kanematsu T, Matsumata T, et al. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis: Further evaluation of limited hepatic resection. Ann Surg 1989;209:297-301. [PubMed]
- Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology 1989;9:457-60. [PubMed]
- Shi M, Zhang CQ, Zhang YQ, et al. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg 2004;28:376-81. [PubMed]
