A comparative study between Embosphere® and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST
Introduction
Gastrointestinal stromal tumors (GISTs) are one of the most common mesenchymal tumors, and account for approximately 2% of gastrointestinal tract tumors (1,2). The liver is the most common site for metastasis from GIST, with a reported incidence of 55-72% in patients with tumor recurrence, and metastatic liver disease is a major determinant of patient survival (3,4). Some studies (5-7) have shown favorable results of transcatheter arterial chemoembolization (TACE) for GIST with liver metastases. At present, there is no standard treatment for metastatic GIST after imatinib and/or sunitinib failure. But a few studies (8,9) have confirmed the role of TACE in the treatment of patients with GIST after failure of tyrosine kinase inhibitors (TKIs).
TACE is primarily used to treat patients with GIST hepatic metastasis who are not suitable candidates for curative treatment (10,11). The rationale for TACE is that intra-arterial chemotherapy with lipiodol and chemotherapeutic agents, followed by selective vascular embolization, will result in a strong cytotoxic effect combined with ischemia [conventional TACE (cTACE)] (12). Recently, another new embolization material Embosphere® (Embospheres, Biosphere Medical, Rockland, MA, USA) has provided a new option for transcatheter embolization. Embosphere® consists of nonabsorbable hydrophilic particles that are calibrated precisely by size. It has the ability to actively reach the microcirculation more effectively compared with conventional, lipiodol-based regimens. Researchers from western countries have also suggested that embolization with these particles offers a superior effect as compared with bland embolization or cTACE for tumors with liver metastasis (13-15).
To date, limited data are available in Asia regarding the use of TACE with Embosphere® for liver metastasis from GIST. We have previously reported preliminary results regarding the use of cTACE in treatment of patients with GIST after failure of TKIs (9). Transcatheter arterial embolization (TAE) with Embosphere® (Embosphere®-TAE) is a new option for these cases and has shown significantly objective response (OR) rates. In the present study, we evaluated the efficacy, safety, and overall survival (OS) benefit of Embosphere® treatment in comparison with cTACE in patients with GIST with liver metastasis after failure of TKIs.
Materials and methods
Study design
The study was an open, retrospective, controlled cohort analysis. In total, 45 patients with GIST and hepatic metastasis who were treated with TACE-based therapy from Aug 2009 to Feb 2013 at the Beijing Cancer hospital of China were included. The median duration of follow-up was 20 months (range, 5-40 months). Four patients were lost follow-up of survival time because of losing contact. The eligibility criteria were as follows: (I) patients with histologically confirmed CD117-positive GIST with liver metastasis who were resistant and/or intolerant to imatinib and/or sunitinib and received TACE for at least one treatment cycle; (II) presence of at least two GIST liver metastatic lesions with a minimum diameter of 10 mm by liver dynamic computed tomography (CT) and target lesions with suitability for accurate repeated measurement; (III) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 with preserved liver function (Child-Pugh Class A or B); and (IV) no previous TACE. Patients with potentially resectable or ablative lesions but at a high risk for surgery and radiofrequency ablation (RFA) were also enrolled. The exclusion criteria included the presence of another primary tumor, advanced liver disease [bilirubin levels >3 mg/dL, and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >5× the upper limit of normal]. The patients with GIST who underwent TAE with Embosphere® (n=19) were compared with controls who received cTACE (n=26). The study was approved by the institutional Ethics Review Board and conducted in compliance with the Declaration of Helsinki.
Treatment response
The treatment response was evaluated three months after receiving TACE using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (16). A complete response (CR) was defined as disappearance of any intra-tumoral arterial enhancement in all the lesions; a partial response (PR) was defined as a 30% decrease in the sum of diameters of viable (contrast enhancement in the arterial phase) lesions; progressive disease (PD) was defined as an increase of 20% in the sum of diameters of viable lesions; and stable disease (SD) was defined as any case that did not qualify as either PR or PD. An OR rate was defined as complete plus partial response.
Statistical analysis
All the statistical analyses were performed using the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Tumor responses and variables between the two groups were compared using χ2, Fisher’s exact, or independent t-tests, as appropriate. Progression-free survival (PFS) and OS curves were constructed using the Kaplan-Meier method and compared with a log-rank test. Statistically significant factors in univariate analysis were estimated using the multivariate Cox proportional hazard model. P<0.05 was considered statistically significant.
Results
Clinical characteristics of patients
The study included 33 males and 12 females. The mean age was 53 years [95% confidence interval (95% CI): 50.8-59.2]. All patients were assessed at registration to have the ECOG performance status grade 0-2. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, 71.1% of the patients were in stage A and 28.9% were stage B; 36% had extrahepatic metastasis. All patients had received sunitinib and/or imatinib prior to TACE or best supportive care (BSC)/TKI introduction treatment. The clinical characteristics of the 45 patients in the two groups are shown in Table 1.
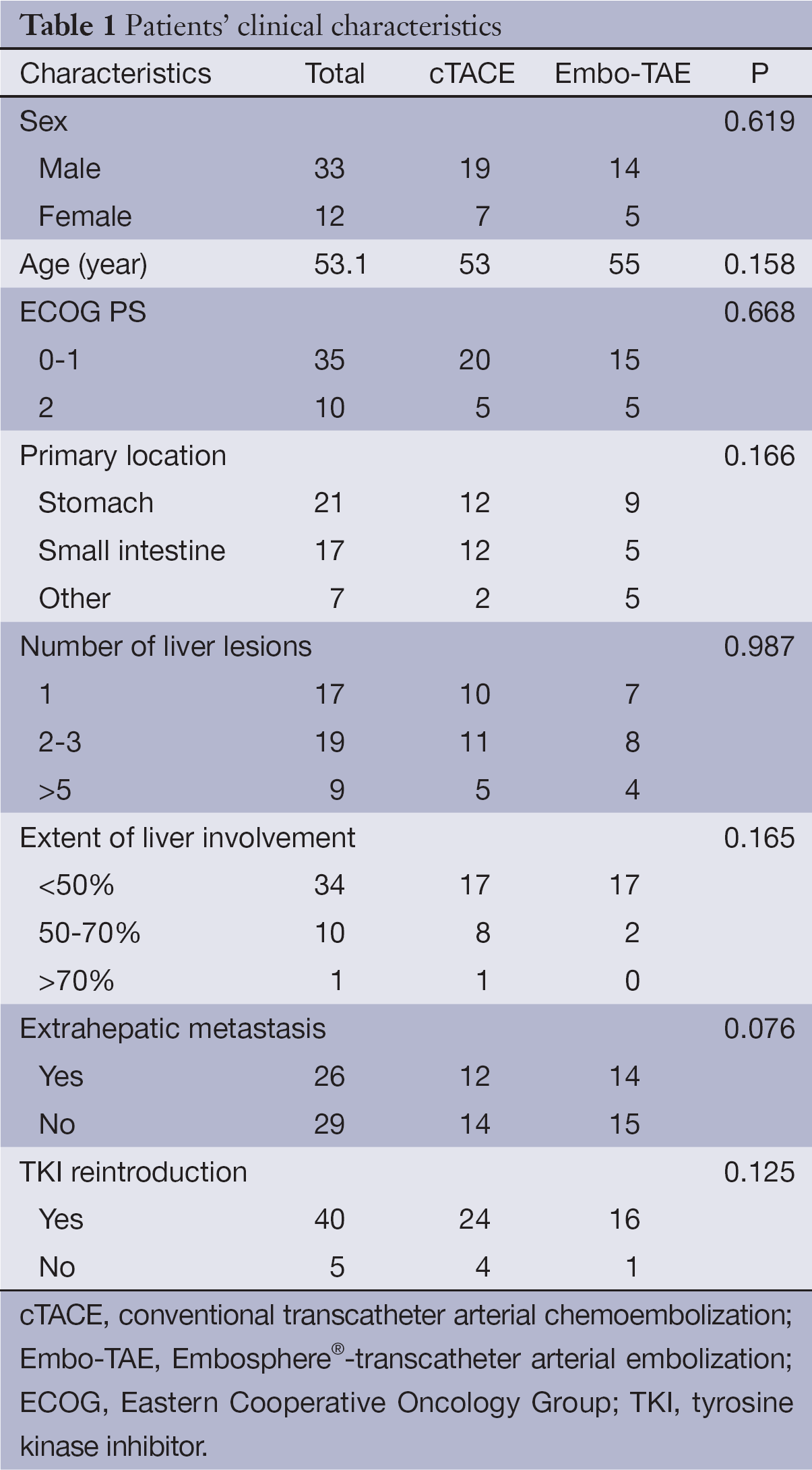
Full table
Treatment in TACE group
The eligibility criteria for TACE included a well-preserved hepatic and renal function, Child-Pugh classification A and B, adequate hematologic function, and ECOG performance status of 0-2. Patients with high-risk factors, such as portal vein occlusion, no hepatopetal flow, passive ascites, encephalopathy, or active cardiac failure, were excluded. Local anesthesia was given with 1% lidocaine. After the introduction of a selective catheter through the femoral artery using the Seldinger technique, the localization of the hepatic arteries was checked with celiac and mesenteric arteriography. This was performed to define the vascular anatomy. Next, an indirect portography was performed to outline the portal circulation in the venous phase. A 5-French catheter was placed in the celiac trunk to identify the hepatic artery. Depending on the size, location, and arterial supply to the tumor, a micro-catheter was advanced further into the segmental feeding arteries to perform embolization. An emulsion containing 5-20 mL iodized oil and 40-80 mg doxorubicin hydrochloride was used depending on the tumor size. Additional embolization was performed using 1-2 mm diameter gelatin sponge particles according to the status of the blood supply. The ideal embolization end-point was the stasis of flow in the tumor-feeding branches. A follow-up abdominal imaging (CT) was performed two months after the first embolization. The follow-up images were assessed by two radiologists and compared with the baseline images to assess the response.
Treatment in Embosphere®-TAE (Embo-TAE) group
Patients in the Embo-TAE group were treated with Embosphere® with a maximum dose of 10 mL (diameter 100-300 lm, 300-500 lm, 500-700 lm). A repeat treatment was scheduled within two weeks after follow-up imaging if there was a residual viable tumor. If available, Embo-TAE was repeated until the occurrence of symptomatic progression, extrahepatic spread, vascular invasion, or development of liver failure, in spite of tumor progression of the target lesions or development of new lesions. When the progressed tumor was not treatable by Embo-TAE or cTACE, patients were treated with imatinib or sunitinib. During the follow-up period, laboratory tests, including albumin, bilirubin, AST, ALT and prothrombin time, and a dynamic CT scan of the liver (nonenhanced, arterial, portal, and delayed venous phases) were performed to evaluate treatment response and preserved liver function every four weeks after treatment. After achieving a CR, treatment responses were assessed using imaging studies every three months.
TKI reintroduction
Almost all the patients (40/45) received standard TKI reintroduction during the intermittent period of TACE. Patients received imatinib (400 mg/d) and/or sunitinib (37.5 mg/d). The interval between TKI therapy and TACE was two weeks.
Treatment response rate
All the patients had measurable metastatic disease according to the mRECIST. Treatment responses were evaluated every 8-10 weeks after TACE. In the Embo-TAE group, 6 (31.6%) and 10 (52.6%) patients showed CR and PR, respectively, 3 (15.8%) had SD, and no patient had PD. In the cTACE group, 1 (3.8%) showed CR, 17 (65.4%) and 6 (23.1%) patients showed PR and SD, respectively, and 2 (7.7%) had PD. Therefore, the treatment response in the Embo-TAE group was significantly higher than that in the cTACE group (P<0.001).
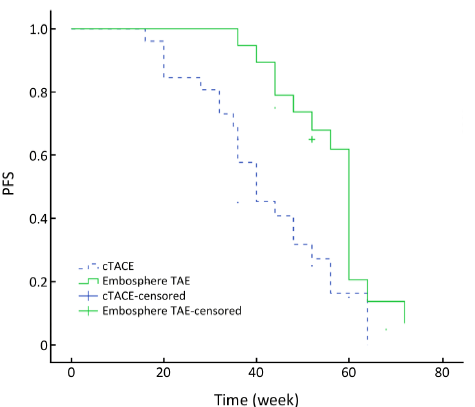
PFS
As of May 2013, 22 (84.6%) patients in the cTACE group had progression of liver metastasis. In the Embo-TAE group, 15 (78.9%) patients had tumor progression. The median PFS in the Embo-TAE group (56.6 weeks, 95% CI: 51.8-61.4 weeks) was longer than that in the cTACE group (42.1 weeks, 95% CI: 36.3-48.0 weeks) (P=0.003, Figure 1).
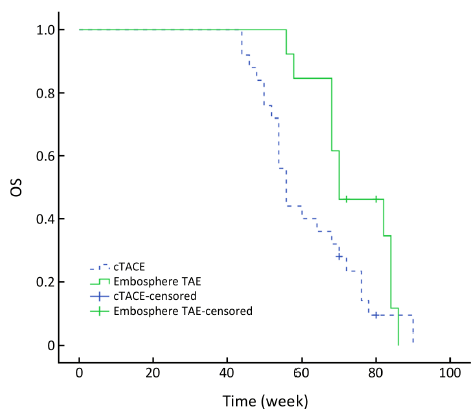
OS
As of May 2013, totally four patients were alive, two patients from the Embo-TAE group and two patients from the cTACE group. All deaths occurred because of tumor progression or related complications. The median OS in the Embo-TAE group was longer than that in the cTACE group (74.0 weeks, 95% CI: 68.2-79.8 vs. 61.7 weeks, 95% CI: 56.2-67.2 weeks) (unadjusted P=0.045, Figure 2). Embo-TAE significantly reduced the risk of death in patients with GIST with liver metastases according to the Cox proportional hazards regression model [hazard ratio (HR): 0.149; 95% CI: 0.064-0.475].
Adverse events
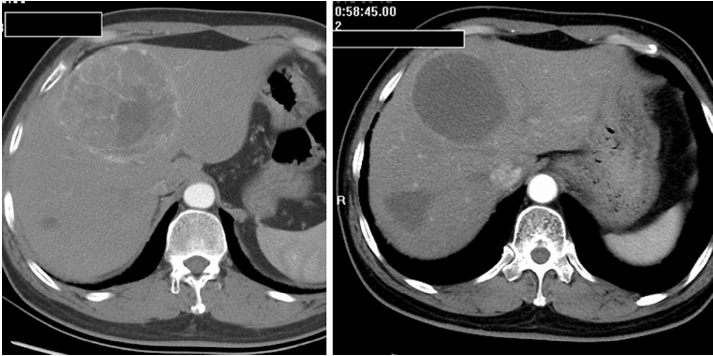
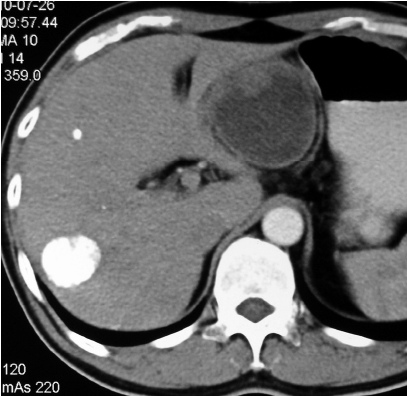
Most patients in the TACE group developed post-embolization complications, which included abnormal liver function, abdominal pain, fever, nausea and so on within one month (Table 2). There were no major complications or grade 3-4 liver toxicities in either group within one month. Increases in ALT after the procedure were significantly less frequent in the Embo-TAE group than in the cTACE group (P=0.0001). The overall frequency of treatment-emergent adverse effects was not different in the Embo-TAE compared with the cTACE group according to National Cancer Institute-Common Toxicity Criteria (NCI-CTC) ver. 3.0. The majority of adverse events in both the groups were in the context of the post-embolization syndrome. Hepatic abscess only occurred in two cases of Embo-TAE group. Treatment with Embo-TAE may have caused complete necrosis, which may lead to hepatic abscess formation more easily (Figures 3,4).
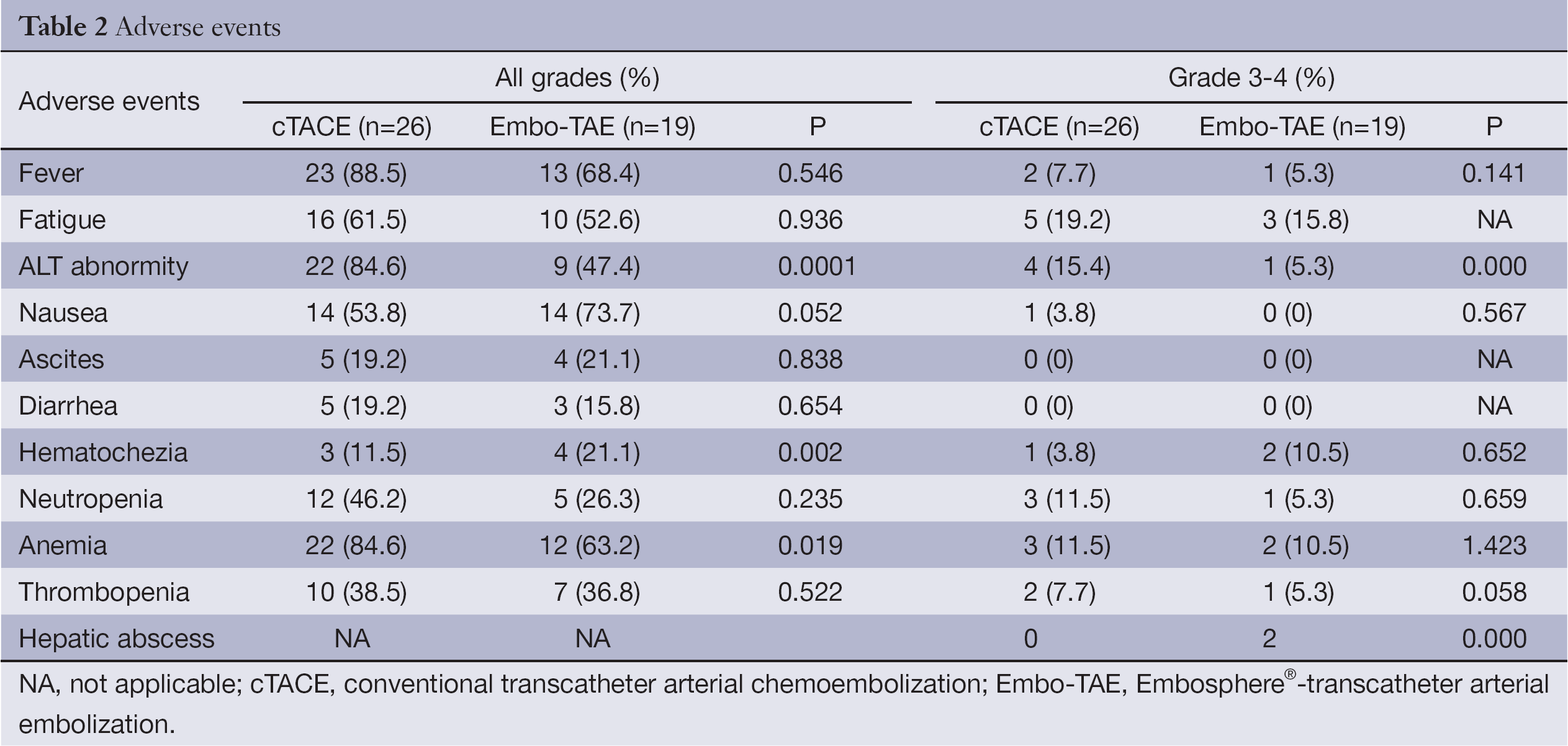
Full table
Discussion
TACE, using lipiodol-mixed chemotherapeutic agent delivered as an emulsion, has been widely used as a standard treatment for hepatocellular carcinoma (HCC) in patients who are not suitable candidates for curative treatment and/or as a bridge to liver transplantation (17). This type of emulsion has been popularly used for years; therefore, it is also called cTACE. Furthermore, it is also regarded as an effective palliative treatment for a series of hepatic metastasis tumors such as neuroendocrine tumors, melanoma, and GIST.
Based on the evidence available at the time, and our own experience, we knew that if either the right or left hepatic artery is occluded, the tissue supplied by the occluded vessel would recruit intrahepatic collateral flow from the contralateral patent vessel virtually immediately (18). In addition, with passage of time after cTACE, the emulsion (lipiodol mixed chemotherapeutic agent) would be washed away and local recurrence of tumor may be discovered. These are the shortcomings associated with cTACE. An important meta-analysis of randomized embolization trials for HCC written by Cammà et al. was published in 2002 (19). Eighteen randomized controlled trials conducted between 1980 and 2000 were included in the analysis. They concluded that chemoembolization significantly reduced the overall 2-year mortality rate compared with nonactive treatment, overall mortality was significantly lower in patients treated with TAE than in those treated with transarterial chemotherapy, and there is no evidence that TACE is more effective than TAE (odds ratio 1.007; 95% CI: 0.79-1.27; P=0.95), which suggests that the addition of an anticancer drug did not improve the therapeutic benefit.
Thereafter, a series of particle embolization materials were developed in an effort to enhance terminal vessel blockade, such as polyvinyl alcohol (PVA), drug-eluting beads (DEB), micron Embozene (Embozene Color Advanced Microspheres; CeloNova BioSciences, Newman, GA, USA), and calibrated spherical hydrophilic particles (Embospheres®, Biosphere Medical).
The recent introduction of Embosphere® in China has provided a valuable alternative treatment for patients with nonresectable GIST hepatic metastasis. According to a multicenter randomized controlled trial (20-23), the use of such type of particles for precision TAE resulted in a statistically significant reduction in systemic toxicity compared with cTACE. However, despite the overall trend favoring treatment with particle-TAE over cTACE, a statistically significant superiority in OR rates was observed only in the subgroup analysis of patients with more advanced disease (Child-Pugh B, ECOG 1, bilobar, or recurrent disease). We performed this retrospective case-control study of Embo-TAE vs. conventional lipiodol-based TACE to evaluate the tumor response and safety in 45 patients with GIST hepatic metastasis. The objective tumor response at two months after treatment was significantly higher in the Embo-TAE group, and the liver toxicity was not significantly different between the two groups. Our previous study (9) drew the conclusion that TACE is effective and well tolerated in patients with GIST with liver metastases after TKI failure. In the present study, a group of patients were enrolled to compare the two treatments and evaluate the efficacy of Embo-TAE in Chinese patients. A significant difference in response rates was observed between patients treated with Embosphere® compared with those treated with cTACE. The CR and PR were 6 (31.6%) and 10 (52.6%), respectively, in the Embo-TAE group, and 1 (3.8%) and 17 (65.4%), respectively, in the cTACE group (P<0.001).
Although the response rates in the cTACE group were similar to those observed in previous study (9), the rates in the Embo-TAE group were greater than those observed in the results reported in previous particle study (24).
The median OS in the Embo-TAE group was longer than that in the cTACE group (74.0 weeks, 95% CI: 68.2-79.8 vs. 61.7 weeks, 95% CI: 56.2-67.2) (unadjusted P=0.045, Figure 2). Embo-TAE reduced the risk of death in patients with GIST with liver metastases according to the Cox proportional hazards regression model (HR: 0.149; 95% CI: 0.064-0.475). But the assumption made of OS statistical difference was not observed. The limitation was that this study included only a small number of patients; thus, conclusions regarding the efficacy of Embo-TAE vs. cTACE cannot be drawn. This is in line with results of previous studies (22-24) in which a statistically significant difference was only identified in radiologic rates of response. The role of particle TAE in the treatment remains to be defined.
The results of this study indicated that patients with early-stage (Child-Pugh class A or <50% liver involvement) or small-nodular tumors showed significantly higher OR rates in the Embo-TAE group. Thus, TAE with Embosphere® may be an effective treatment modality for GIST hepatic metastasis.
Furthermore, all the cases enrolled in this study were patients with TKI therapy failure. Almost all cases had already entered into a late stage with a poor liver function reserve. As per experience from this study, a better early treatment response and control of disease progression following Embo-TAE may play a role in the survival benefit by delaying the deleterious effects of tumor progression. Previous studies have shown that the TACE/TAE was significantly better than the BSC control group. The difference in OS rates between the Embo-TAE and cTACE groups was statistically significant only in early-stage GIST (Child-Pugh class A or liver involvement within 50%) compared with late-stage GIST with hepatic metastasis (Child-Pugh class B and C, or liver involvement more than 50%) (P=0.003 and 0.002, respectively). Therefore, patients in early stage survived longer after treatment with Embo-TAE than with cTACE. At the same time, it can be assumed that patients with GIST liver metastasis should receive TAE or TACE as early as possible combined with TKI (imatinib/sunitinib) therapy, just like the combination of sorafenib with TACE to treat the advanced HCC nowadays. Although I may have raised more questions than I’ve answered, I hope that I have at least provided a window into the “when to perform TACE” and “how to choose a TACE protocol (particles TAE vs. conventional bland embolization” debate. Would a randomized controlled trial of the “best” chemoembolization protocol be implementedin the future?
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3:655-64. [PubMed]
- Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 2005;41:2868-72. [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [PubMed]
- Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol 2010;16:2726-34. [PubMed]
- Kobayashi K, Gupta S, Trent JC, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer 2006;107:2833-41. [PubMed]
- Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer 2006;107:1617-23. [PubMed]
- Kobayashi K, Szklaruk J, Trent JC, et al. Hepatic arterial embolization and chemoembolization for imatinib-resistant gastrointestinal stromal tumors. Am J Clin Oncol 2009;32:574-81. [PubMed]
- Kamat PP, Gupta S, Ensor JE, et al. Hepatic arterial embolization and chemoembolization in the management of patients with large-volume liver metastases. Cardiovasc Intervent Radiol 2008;31:299-307. [PubMed]
- Cao G, Li J, Shen L, et al. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol 2012;18:6134-40. [PubMed]
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429-42. [PubMed]
- Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. [PubMed]
- de Baere T, Deschamps F, Teriitheau C, et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol 2008;19:855-61. [PubMed]
- Rajan DK, Soulen MC, Clark TW, et al. Sarcomas metastatic to the liver: response and survival after cisplatin, doxorubicin, mitomycin-C, Ethiodol, and polyvinyl alcohol chemoembolization. J Vasc Interv Radiol 2001;12:187-93. [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlledtrial. Lancet 2002;359:1734-9. [PubMed]
- Ngan H, Lai CL, Fan ST, et al. Transcatheter arterial chemoembolization in inoperable hepatocellular carcinoma: four-year follow-up. J Vasc Interv Radiol 1996;7:419-25. [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [PubMed]
- Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008;19:862-9. [PubMed]
- Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010;33:552-9. [PubMed]
- Padia SA, Shivaram G, Bastawrous S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol 2013;24:301-6. [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [PubMed]
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81. [PubMed]