A case of sudden hearing loss during treatment with decitabine for chronic myelomonocytic leukemia
Introduction
Chronic myelomonocytic leukemia (CMML) is a bone marrow disorder characterized by the overproduction of maturing monocytic cells, and sometimes dysplastic neutrophils. CMML is often accompanied by anemia and/or thrombocytopenia (1). In 2006, the Food and Drug Administration approved decitabine for the treatment of CMML. The non-hematologic side effects of decitabine are minimal and myelosuppression-associated complications are acceptable. Here, we describe one patient with CMML who developed sudden hearing loss (SHL) under decitabine treatment.
Case report
A 73-year-old male patient came to our hospital with knee pain for two weeks. During this hospitalization, he had leukocytosis, with a white blood cell (WBC) count of 36,280/mm3. The differential count showed 53% neutrophils, 3% lymphocytes, 25% monocytes, and 5% blasts. Coagulation studies revealed an activated partial thromboplastin time (aPTT) of 33.7 s and a prothrombin time (PT) of 13.3 s. The patient was transferred to the hematologist. CMML was diagnosed based on a bone marrow biopsy and aspiration (promonocytes, 7.4%; blasts, 4.4%). The patient was started on decitabine (20 mg/m2 for 5 d in a 4-week cycle). After 2 cycles, the WBC count had declined to 14,110/mm3 and the other side effects were minimal.
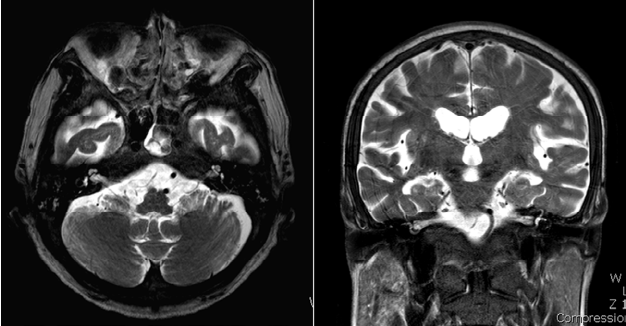
He was hospitalized for the 3rd cycle of decitabine and the laboratory testing showed a total leukocyte count of 22,520/mm3. During the 3rd cycle on the 5th day of chemotherapy, he abruptly complained of difficulty hearing on the left side without dizziness. The physical examination revealed normal external auditory canals with intact tympanic membranes bilaterally. Pure tone audiometry revealed moderate sensorineural hearing loss in the right ear (48 dB HL by 4-tone average) and severe sensorineural hearing loss in the left ear (84 dB HL by 4-tone average; Figure 1). Speech audiometry revealed word discrimination scores of 80% for the right ear and 20% for the left ear. Radiologic evaluation of the temporal region and brain, including magnetic resonance imaging (MRI) scan, revealed no significant abnormalities of the brain and inner ear (Figure 2).
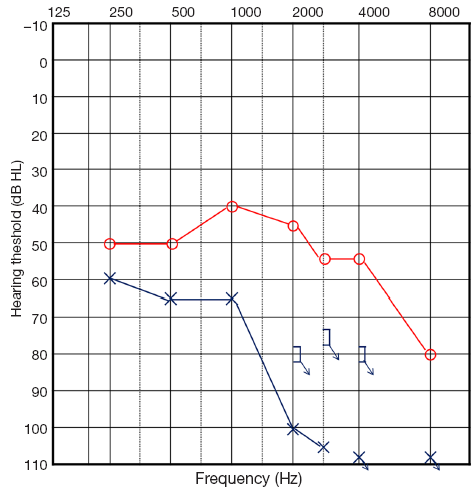
Even though there were no baseline audiologic test results before the onset of symptoms, the patient previously had equal hearing in both ears and the test results were compatible with sensorineural hearing loss in the left ear, thus the patient was diagnosed with SHL based on the history and audiologic testing. At the time of symptom onset, the WBC count was 32,600/mm3, the hemoglobin was 7.6 g/dL, and the platelet count was 339,000/mm3.
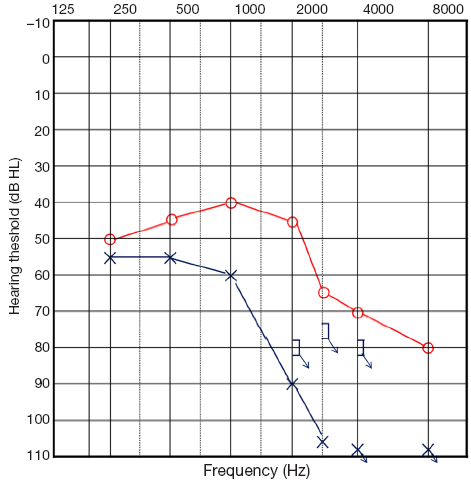
Intratympanic steroids were injected every other day (six times) instead of oral steroids based on the patient’s hematologic status. Carbogen was also inhaled to increase the blood flow and oxygen supply to the injured hair cells of the cochlear and inner ear structures. The patient had follow-up visits and pure tone audiograms were conducted for eight months. During the eight months, treatment was continued to the 8th cycle of decitabine as planned. While a hematologic response was achieved, the WBC count normalized to 9,980/mm3, and pure tone audiometry persistently revealed a similar hearing threshold (49 dB HL in the right ear, 78 dB HL in the left ear by 4-tone average; Figure 3). Speech audiometry revealed word discrimination scores of 96% for the right ear and 32% for the left ear. Despite, hematologic improvement, hearing improvement was not achieved.
Discussion
CMML is a hybrid disorder characterized by proliferation of the myeloid series and dysplasia of the erythroid-megakaryocytic series (2). The incidence of CMML is approximately three cases per 100,000 individuals >60 years of age, and accounts for 12% of all French-American-British (FAB)-defined myelodysplastic syndrome (MDS). The clinical symptoms and other phenotypic manifestations are caused by complications resulting from cytopenia, dysplastic cells that function abnormally, leukemic infiltration of various organ systems, and general constitutional symptoms, such as fever and malaise (3). Otologic findings, such as, SHL, tinnitus, vertigo, facial weakness, and infection, were found in 16-40% of leukemic patients (4). However, SHL is a rare manifestation in CMML patients (4). The pathogenesis of hearing loss in chronic myeloid leukemia (CML) involves multiple mechanisms, such as leukemic infiltration, hyperviscosity syndrome, inner ear hemorrhage, and infection. Hyperleukocytosis results in a slowing of the circulation through the small blood vessels in the brain stem, and is thus as a cause of deafness (5). Cochlear damage is secondary to the anoxia or hypoxia generated and can occur due to permanent and/or temporary vessel occlusion by hyperviscosity, such as multiple myeloma and Waldenstrom's macroglobulinemia (6). Hyperviscosity syndrome often requires leukocyte counts >50,000/mm3.
In our case, there were intermittent normal WBC counts reflecting a hematologic response after decitabine treatment, but leukocytosis continued during the first three months of treatment, with a peak WBC count of 71,180/mm3.
SHL is considered a syndrome, rather than a diagnosis, and is defined as hearing loss of at least 30 dB in 3 sequential frequencies occurring over <3 d (7). There is no standard treatment protocol for SHL, but the most accepted treatment is a tapered course of oral systemic steroids.
Due to the side effects of high-dose systemic steroids, many physicians are hesitant to use systemic steroids for patients who have an underlying chronic disease. Intratympanic dexamethasone (ITD) offers the potential for direct delivery of high concentrations of the steroid to the inner ear, thus avoiding systemic effects (8).
We performed intratympanic steroid injection every other day for a total of six times instead of oral steroid administration based on the patient’s hematologic status (9). Carbogen inhalation is known to cause vasodilation and increase blood flow and oxygen supply to injured hair cells of the cochlear and inner ear structures. We performed carbogen inhalation against the hyperviscosity theory.
Generally, in the case of sudden deafness, there is a reduced likelihood for hearing improvement after six months of symptom onset. The patient presented herein did not show a change in hearing level for eight months; we categorized his SHL as “no improvement” based on Siegel’s criteria.
One theory that explains the cause of SHL involves leukemia-mediated infarction of the inner and middle ears (10). A second theory suggests that hyperviscosity with occlusion of the labyrinthine and other small arteries of the vertebra-basilar area. A third theory focuses on the toxicities of decitabine, even if decitabine-related extramedullary toxicities are uncommon. There are few reports which have addressed ototoxicities of hypomethylating agents, such as decitabine. Indeed, there may be unknown adverse effects due to the short-term clinical experience (four years). There have been no reported cases of SHL in CMML patients treated with decitabine. We have described the first case of SHL in a patient with CMML while being treated with decitabine.
In conclusion, we must verify that idiopathic SHL can occur in CMML patients during treatment with decitabine. The theory that a poorly controlled underlying disease or decitabine can be associated with the causes of irreversible SHL has been confirmed. The patients being treated with decitabine need to be monitored for hearing loss and otologic symptoms.
Acknowledgements
This work was supported by a Chung-Ang University Research Grant. Dr. Park, Dr. Kim and Dr. Moon have no financial disclosures. Dr. Park and Dr. Kim receive institutional research support from Chung-Ang University.
Disclosure: The authors declare no conflict of interest.
References
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982;51:189-99. [PubMed]
- Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer 2007;109:713-7. [PubMed]
- Orazi A, Germing U. The myelodysplastic/myeloproliferative neoplasms: myeloproliferative diseases with dysplastic features. Leukemia 2008;22:1308-19. [PubMed]
- Acar GO, Acioglu E, Enver O, et al. Unilateral sudden hearing loss as the first sign of chronic myeloid leukemia. Eur Arch Otorhinolaryngol 2007;264:1513-6. [PubMed]
- Resende LS, Coradazzi AL, Rocha-Junior C, et al. Sudden bilateral deafness from hyperleukocytosis in chronic myeloid leukemia. Acta Haematol 2000;104:46-9. [PubMed]
- Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol 2006;72:554-61. [PubMed]
- Flint PW, Haughey BH, Lund VJ, et al. Cummings Otolaryngology-Head and Neck Surgery. 5th Ed. Mosby, 2010.
- Chandrasekhar SS. Intratympanic dexamethasone for sudden sensorineural hearing loss: clinical and laboratory evaluation. Otol Neurotol 2001;22:18-23. [PubMed]
- Moon IS, Lee JD, Kim J, et al. Intratympanic dexamethsone is an effective method as salvage treatment in refractory sudden hearing loss. Otol Neurotol 2011;32:1432-6. [PubMed]
- Naithani R, Chandra J, Mathur NN, et al. Hearing loss in chronic myeloid leukemia. Pediatr Blood Cancer 2005;45:54-6. [PubMed]
