The evolving Gleason grading system
Historical overview of the Gleason grading system
The Gleason grading system for prostate adenocarcinoma originated in the 1960s–1970s from a pioneering randomized, well-controlled, prospective study initiated by the Veterans Administration of the USA, in which over 2,900 patients were included. Dr. Donald Gleason detailed and summarized the histological growth patterns (grades) of prostate adenocarcinoma, and the correlation with clinical data such as staging and prognosis were analyzed. The eponymous Gleason patterns have since been well-known, although a number of other grading systems have also been proposed or used over the last few decades. The WHO endorsed the Gleason grading system in the 2004 classification of prostate cancer, which has also been incorporated into the AJCC/UICC staging system, as well as the NCCN guidelines as one of the key factors [together with serum prostate specific antigen (PSA) and staging] in treatment decision (1-3).
The classical Gleason system defines five histological growth patterns (grades). Gleason 1 represents the best differentiated and is correlated with the most favorable prognosis, whereas Gleason 5 the least differentiated and correlated with poor prognosis. As many prostate adenocarcinomas harbored two or more Gleason patterns, the Gleason score was developed, which was shown to correlate with the biological behavior of prostate adenocarcinoma even better. The sum of the primary (e.g., Gleason 3) and secondary (e.g., Gleason 4) patterns (grades) yields the Gleason score (e.g., Gleason score =3+4=7). In those cases with just one pattern, e.g., 3, the primary and secondary patterns are considered the same, yielding a Gleason score of 3+3=6.
The basic principles of classical Gleason grading (3-6)
To fully understand the current practice of Gleason grading, it would be helpful to first review the original Gleason grading system, which is based on architectural patterns of prostate adenocarcinoma seen on H&E sections, rather than cellular features. In the original system, Gleason pattern 1 is characterized by a well-circumscribed, nodular lesion composed of roughly uniform, closely compacted, discrete, well-differentiated glands of moderate size. It has been recognized that this “pattern” is extremely rare, if not non-existent, and many of the ones defined by Dr. Gleason in his original study may be mimicking lesions [such as adenosis or atypical adenomatous hyperplasia (AAH)] rather than bona fide prostate cancer. In contrast, Gleason pattern 2 may show variations in sizes of the neoplastic glands, slightly increased stroma between the glands, and even slight irregularity at the periphery of the nodule. This pattern is currently also considered to be very rare. Gleason pattern 3 has been seen in many series as the most common pattern, comprised of individual, discrete and distinct neoplastic glands, typically small, but often of variable sizes and infiltrating into the stroma in between the benign glands.
The original pattern 3 included cribriform structures, some with irregular contours, and some within small, smooth, rounded glandular spaces, or “gland in gland formation”, i.e., glomerulation, which have been subsequently moved into pattern 4 in the most recent proposals (see below). Gleason pattern 4 features fused glands, which are no longer individual or distinct, resulting in broad, irregular fused glandular or cribriform patterns. A variant is the hypernephroid pattern with sheets of cells with abundant clear cytoplasm. Comedo type necrosis in the context of these structures leads to upgrading to pattern 5, which also includes solid or cordlike growth or infiltration by individual tumor cells, without any trace of gland formation (6).
The modified Gleason system based on the 2005 International Society of Urologic Pathology (ISUP) Consensus and later developments
Many changes since the 1960s–1970s have called for updating of the original Gleason system. For example, new growth patterns or variants of prostate adenocarcinoma have been recognized, which need to be incorporated into the system. Modified needle biopsy protocols and modern surgical approaches in combination with increased screening by serum PSA and other modalities yielded samples which required pathologists to address many issues such as grading multiple core biopsies from different sites or multiple nodules in radical prostatectomies (RP). These also raised such issues as how to interpret and score biopsies with tertiary (in addition to the primary and secondary) patterns, and how to differentiate between cribriform patterns in well-defined spaces from high grade PIN, which have been better appreciated with the availability of basal cell immunohistochemical markers.
The most important advance in this regard is the ISUP consensus published in 2005, which represented the culmination of years of attempts by pathologists to address controversial issues and to reach consensus in the Gleason grading system. The consensus resulted from discussions by over 70 leading urologic pathologists, and “consensus” was defined as agreement by at least two thirds of the participants. Some of the most important updates in the 2005 consensus are highlighted below (7-15).
One of the most prominent changes in the consensus is that Gleason score 1+1=2 should not be diagnosed, despite the allocation for very rare exceptions. It has been recognized that diagnosing Gleason 1 on needle biopsies is not acceptable, since a “Gleason 1” nodule cannot be assessed by a core biopsy. Even with TURP or RP samples, the original Gleason score 1+1=2 nodules mostly are adenosis (AAH) by modern standards. This basically eliminates Gleason 1 pattern.
Gleason score 3 or 4 on needle biopsies (comprised of grades 1+2, 2+1 and 2+2) has also been controversial, given its poor reproducibility and poor correspondence with the grading on later prostatectomy samples. Similar to the original Gleason 1, the edge of the so-called Gleason 2 nodule can hardly be properly assessed on needle biopsies. The ISUP consensus recommended that diagnosis of Gleason score 3 or 4 be made only “rarely, if ever”, for example, on needle biopsies from transition zone or apex, best with consultation with experts. Some urologic pathologists would diagnose Gleason score 2+3=5 or 3+2=5 if the edge of the nodule shows slight irregularity (12).
The original Gleason pattern 3 actually included diverse patterns, ranging from the classical individual, distinct glands of variable sizes most characteristic of this pattern, to cribriform growths, and even to individual cells. The 2005 consensus addressed the issue of the controversial cribriform Gleason pattern 3, and unanimously agreed to remove individual cells, as well as to move large cribriform growths into pattern 4, but still allowed diagnosis of cribriform pattern 3 in well-circumscribed, smooth and rounded glands the size of normal glands. Some urologic pathologists required other features to diagnose cribriform pattern 3, such as evenly spaced lumina and even thickness of inter-connecting bridges.
Thus, based on the 2005 consensus, most cribriform patterns would have been placed into Gleason pattern 4, but allowance for rare cribriform pattern 3 was still made (12). However, additional data, experiences accumulated in larger centers, and discussions by urological pathologists (post-ISUP consensus conference) further led to the proposal that all cribriform glands should be considered Gleason pattern 4 (16). The most recent AFIP fascicle on tumors of the prostate gland now published by the ARP press (17) and other monographs (18) promoted such modifications, which have been adopted by most practicing urologic pathologists.
The consensus also addressed the issue of “ill-defined glands with poorly formed glandular lumina”. A cluster of such glands should be classified as Gleason pattern 4, as it is unlikely to represent tangential sections of Gleason pattern 3 glands, which need to be ruled out. However, very small but distinct glands still should be assigned Gleason pattern 3. Comedo necrosis in solid nests or cribriform background formations, represents Gleason pattern 5, and it was emphasized that stringent criteria should be applied for comedo necrosis, which include intraluminal necrotic cells and karyorrhexis.
The consensus also addressed the issues related to grading other structural features as well as variants of prostate adenocarcinoma. One of these was adenocarcinoma with vacuoles. As vacuoles can be observed not only in Gleason pattern 4 (more commonly) but also in Gleason pattern 5 and Gleason pattern 3 tumors (rarely), it was proposed that vacuoles should be ignored and that the grading should be based only on the underlying structural patterns. Similarly, focal mucinous extravasation as well as mucinous fibroplasia (collagenous micronodules) should be ignored and the grading should be based on the underlying gland structures. At the consensus, the opinions regarding the grading of glomeruloid structures were divided, but later discussions led to their inclusion in pattern 4.
For grading foamy gland carcinomas, the foamy cytoplasm is to be ignored and the grading should be based on the underlying structures. Consensus was also reached to grade pseudohyperplastic adenocarcinoma as Gleason score 3+3=6, and ductal adenocarcinoma as Gleason score 4+4=8. However, the opinions regarding the grading of colloid carcinomas were divided (grade as Gleason score 8, or ignore the extracellular mucin and grade according to underlying structures).
In summary, modified Gleason system based on the 2005 consensus and later developments basically eliminated Gleason grade 1, and put very stringent limits on Gleason pattern 2. Gleason 3 would thus be the lowest grade assigned if no higher grade patterns are identified. Many changes were made to Gleason pattern 3, particularly the moving of most original Gleason pattern 3 cribriform structures as well as clusters of poorly formed glands into Gleason 4 (7).
Impact and clinicopathological correlations of the modified Gleason grading system
The most direct impact of the modified Gleason system is a shift toward higher Gleason score being reported. In a review of 97,168 patients newly diagnosed of prostate cancers on needle biopsy in Sweden from 1998 to 2011, it was found that, after standardization for stage and PSA, there was an increase of Gleason score 7–10 diagnoses from 59% to 72% [2011]. Among low-risk cases (clinical stage T1 and serum PSA 4–10 ng/mL) the increase was from 16% to 40%, whereas among high-risk cases (stage T3 and PSA 20–50 ng/mL) the increase was from 65% to 94%. At the same time, diagnoses of Gleason score 2–5 decreased from 27% to 1%, whereas Gleason score 2–4 was almost discontinued (19). In another series of thin core biopsies, re-grading by the 2005 ISUP criteria resulted in increase of score 7 tumors from 25.5% to 67.9% (20). These changes resulted in increase of high-risk-category tumors, from 31.3% to 41.1% of cases according to some studies (21).
There are data showing that the overall agreement between grading of needle biopsy and radical prostatectomy specimens increased (e.g., from 58% to 72%) after adoption of the modified system, particularly for biopsies with Gleason score of 3+4=7 (88%) (20). However, other studies appeared to show no significant change in level of agreement between scores of needle biopsies and subsequent radical prostatectomy specimens (22).
Most studies addressing the issue whether the modified system correlated with clinical stage and patient outcome better than the original scheme have reached positive conclusions, despite occasional dissent (23,24). For example, the modified system, particularly relocation of original Gleason pattern 3 cribriform growth into Gleason pattern 4, has shown to be valuable in predicting biochemical recurrence after radical prostatectomy (21,25-28).
In a review of 1,240 consecutive radical prostatectomy cases, 34% of patients with classical Gleason score 3+3=6 cancer were upgraded to modified Gleason score 7 or 8, who were shown to be at intermediate risk between patients with modified Gleason score 3+3=6 and those with classical Gleason score 3+4=7 (29).
However, upgrading classical Gleason 3+3=6 cancers to Gleason 3+4=7 when minimal quantity of Gleason pattern 4 component was identified by the modified Gleason criteria of ISUP also met some questioning. For example, in a study of 107 biopsies with GS 3+4=7 out of 256 consecutive needle biopsies with corresponding radical prostatectomy specimens, 22 cases (20.6%) showed only minimal quantities of modified Gleason 4 pattern, and 10 of the 22 cases (45%) had insignificant tumor in the radical prostatectomy specimen (30). These authors observed that the Gleason score, pathologic stages, total tumor volume, and insignificant tumor rate in radical prostatectomy in the biopsy group of Gleason 3+3=6 were similar to that in Gleason 3+ minimal 4=7, but different from GS 3+4=7 if Gleason 4 reached 6% to 50%. Interestingly, the latter two groups also showed significant differences. These authors argued that the clinical significance of Gleason 3+4=7 with minimal Gleason 4 in biopsies needed further evaluation (30).
An important aspect for any grading system to be clinically useful is its intra- and inter-observe reproducibility. Intraobserver agreement on Gleason scores has been reported to vary from 43% to 78% (31,32), whereas interobserver agreement have been reported to vary from 36% to 81% for exact agreement, and 69% to 86% when deviation within one Gleason score unit was considered to be in agreement (33). Similarly, the modified system, particularly the new definition of Gleason pattern 4 and the actual decrease in the number of patterns (since some diagnostic categories were basically abandoned), appeared to have led to improved overall interobserver reproducibility, rising to about 80% (14,34-36).
Reporting Gleason grade/score
It is essential to recognize the basic rules of the modified Gleason system, as well as the differences in reporting on different types of samples.
For needle biopsies, identifiable high-grade component of any quantity should always be included in the Gleason score, as it indicates high probability of finding significant high-grade tumor in the prostate. In contrast, lower-grade patterns occupying less than 5% of the tumor should be ignored (the 5% cut-off rule). In addition to the basic operation of summing up the primary and the secondary patterns to yield the Gleason score, one must remember that when tertiary component (i.e., occupying the smallest percentage when three patterns are present) is identified, but is of the highest grade among the three in needle biopsy, this component should be included as the second grade. That is, Gleason score = primary pattern + the highest pattern, in this scenario. For example, in a biopsy with Gleason patterns 3 (80%), 4 (15%), and 5 (5%), the Gleason score should be 3+5=8, rather than 3+4=7. For multiple biopsy cores with different grades, grading individual cores has been recommended, as long as the anatomical sites of the cores could be identified (by submission in separate containers and separate embedding/sectioning, or by inking in different colors), whereas an overall score may also be provided (3,6,12).
For radical prostatectomy specimens and TURP samples, the basic rules (Gleason score = primary + secondary patterns, and the 5% cut-off rule for lower-grade secondary pattern) apply. It differs from reporting needle biopsies in that tertiary, highest grade should be reported separately, preferably with an accompanying note, rather than incorporated into the Gleason score. Also separate Gleason score should be assigned to each dominant tumor nodule in cases of multiple nodules with different grades. Rarely, a nondominant nodule may show a higher score, and the grade for this nodule should be reported separately, because it likely will drive the biologic behaviour of the cancer.
Future directions and developments
An international consensus meeting to update Gleason grading convened in Chicago (US) in late 2014, which represented not only experts in pathology, but also urologists, radiation and medical oncologists (37-39). The meeting was conducted by the ISUP in response to the need to address issues not resolved or not covered in the 2005 consensus, as well as new research data and challenges from clinicians to the current grading system.
The meeting reached consensus on several major issues, including that cribriform and glomeruloid glands should be graded as Gleason 4, irrespective of morphology, and that mucinous (colloid) carcinoma of the prostate should be graded on the basis of underlying growth patterns (rather than all as pattern 4). The consensus emphasized that intraductal carcinoma of the prostate in the absence of invasive component should not be graded but should be commented on in the report to alert clinicians of its consistent association with aggressive cancer. The meeting reached consensus on many issues regarding various morphologies within Gleason patterns 3, 4, and 5. Reporting of percentage of Gleason 4 component in Gleason score 7 (particularly Gleason score =3+4) tumors is also a major recommendation, as it may have major impact on treatment decisions (37-39).
The most important development of the meeting is the proposal of a new prognostic grade grouping system, which may have major impact on practicing pathologists and clinicians. In this new system, Gleason scores less than or equal to 6 are to be lumped into prognostic grade group I, Gleason score 3+4=7 to group II, Gleason score 4+3=7 to group III, Gleason score 4+4=8 to group IV, and Gleason score 9-10 to group V (37-39). This is basically a new grading system, although it is based on Gleason patterns, together with which it is to be used for the time being. Since the new “Grade Group” system has been incorporated into the new edition of World Health Organization classification of prostate tumors (released in January 2016) (40), clear understanding of the system by both pathologists and clinicians is essential.
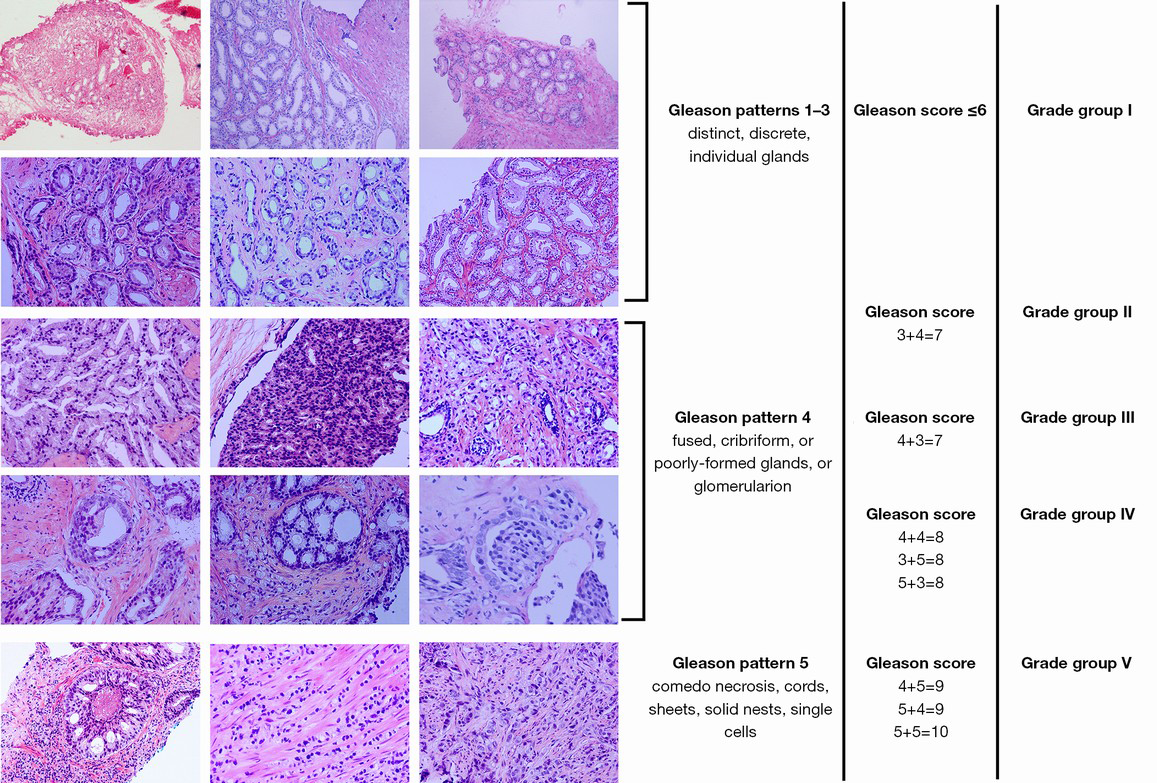
The current Gleason grading system with typical Gleason patterns and the new Group Grade system are illustrated in Figure 1.
Acknowledgements
Funding: The authors are supported by grants from the Natural Science Foundation of China (NSFC 81272848, 81272820, 81302225, 81572540).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gleason DF. Histologic grading and clinical staging of prostate carcinoma. In: Tannenbaum M, editor. Urologic pathology: the prostate. Philadelphia: Lea & Fibiger, 1977:171-98.
- Kovi J, editor. Surgical pathology of the prostate. Boca Raton: CRC Press Inc., 1989:195-200.
- Eble JN, Sauter G, Epstein JE, et al, editors. World Health Organization Classification of Tumours. Pathology and genetics of the urinary system and male genital organs. Lyon: IARC Press, 2004:159-215.
- Humphrey P, editor. Prostate pathology. Chicago: ASCP Press, 2003:338-75.
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17:292-306. [PubMed]
- Amin MB, Grignon DJ, Humphrey PA, editors. Gleason grading of prostate cancer. A contemporary approach. Philadelphia: Lippincott Williams & Wilkins, 2004:1-116.
- Shah RB. Current perspectives on the Gleason grading of prostate cancer. Arch Pathol Lab Med 2009;133:1810-6. [PubMed]
- Osunkoya AO. Update on prostate pathology. Pathology 2012;44:391-406. [PubMed]
- Lotan TL, Epstein JI. Clinical implications of changing definitions within the Gleason grading system. Nat Rev Urol 2010;7:136-42. [PubMed]
- Helpap B, Egevad L. Modified Gleason grading. An updated review. Histol Histopathol 2009;24:661-6. [PubMed]
- Epstein JI, Allsbrook WC Jr, Amin MB, et al. Update on the Gleason grading system for prostate cancer: results of an international consensus conference of urologic pathologists. Adv Anat Pathol 2006;13:57-9. [PubMed]
- Epstein JI, Allsbrook WC Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005;29:1228-42. [PubMed]
- Epstein JI. An update of the Gleason grading system. J Urol 2010;183:433-40. [PubMed]
- Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med 2012;136:426-34. [PubMed]
- Delahunt B, Miller RJ, Srigley JR, et al. Gleason grading: past, present and future. Histopathology 2012;60:75-86. [PubMed]
- Latour M, Amin MB, Billis A, et al. Grading of invasive cribriform carcinoma on prostate needle biopsy: an interobserver study among experts in genitourinary pathology. Am J Surg Pathol 2008;32:1532-9. [PubMed]
- Epstein JI, Cubilla AL, Humphrey PA, editors. Tumors of the prostate gland, seminal vesicles, penis, and scrotum. Washington DC: The ARP Press, 2011.
- Epstein JI, editor. The Gleason grading system. A complete Guide for Pathologists and Clinicians. Philadelphia: Wolters Kluver Health | Lipincott Williams & Wilkins, 2013.
- Danneman D, Drevin L, Robinson D, et al. Gleason inflation 1998-2011: a registry study of 97,168 men. BJU Int 2015;115:248-55. [PubMed]
- Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Arch 2006;449:622-7. [PubMed]
- Delahunt B, Lamb DS, Srigley JR, et al. Gleason scoring: a comparison of classical and modified (international society of urological pathology) criteria using nadir PSA as a clinical end point. Pathology 2010;42:339-43. [PubMed]
- Zareba P, Zhang J, Yilmaz A, et al. The impact of the 2005 International Society of Urological Pathology (ISUP) consensus on Gleason grading in contemporary practice. Histopathology 2009;55:384-91. [PubMed]
- Helpap B, Egevad L. Correlation of modified Gleason grading with pT stage of prostatic carcinoma after radical prostatectomy. Anal Quant Cytol Histol 2008;30:1-7. [PubMed]
- Naito S, Kuroiwa K, Kinukawa N, et al. Validation of Partin tables and development of a preoperative nomogram for Japanese patients with clinically localized prostate cancer using 2005 International Society of Urological Pathology consensus on Gleason grading: data from the Clinicopathological Research Group for Localized Prostate Cancer. J Urol 2008;180:904-9; discussion 909-10. [PubMed]
- Billis A, Guimaraes MS, Freitas LL, et al. The impact of the 2005 international society of urological pathology consensus conference on standard Gleason grading of prostatic carcinoma in needle biopsies. J Urol 2008;180:548-52; discussion 552-3. [PubMed]
- Uemura H, Hoshino K, Sasaki T, et al. Usefulness of the 2005 International Society of Urologic Pathology Gleason grading system in prostate biopsy and radical prostatectomy specimens. BJU Int 2009;103:1190-4. [PubMed]
- Billis A, Quintal MM, Meirelles L, et al. The value of the 2005 International Society of Urological Pathology (ISUP) modified Gleason grading system as a predictor of biochemical recurrence after radical prostatectomy. Int Urol Nephrol 2014;46:935-40. [PubMed]
- Kir G, Sarbay BC, Gümüş E, et al. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol Res Pract 2014;210:640-4. [PubMed]
- Dong F, Wang C, Farris AB, et al. Impact on the clinical outcome of prostate cancer by the 2005 international society of urological pathology modified Gleason grading system. Am J Surg Pathol 2012;36:838-43. [PubMed]
- Huang CC, Kong MX, Zhou M, et al. Gleason score 3 + 4=7 prostate cancer with minimal quantity of gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am J Surg Pathol 2014;38:1096-101. [PubMed]
- Griffiths DF, Melia J, McWilliam LJ, et al. A study of Gleason score interpretation in different groups of UK pathologists; techniques for improving reproducibility. Histopathology 2006;48:655-62. [PubMed]
- Melia J, Moseley R, Ball RY, et al. A UK-based investigation of inter- and intra-observer reproducibility of Gleason grading of prostatic biopsies. Histopathology 2006;48:644-54. [PubMed]
- Lopez-Beltran A, Mikuz G, Luque RJ, et al. Current practice of Gleason grading of prostate carcinoma. Virchows Arch 2006;448:111-8. [PubMed]
- Steinberg DM, Sauvageot J, Piantadosi S, et al. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol 1997;21:566-76. [PubMed]
- Fine SW, Epstein JI. A contemporary study correlating prostate needle biopsy and radical prostatectomy Gleason score. J Urol 2008;179:1335-8; discussion 1338-9. [PubMed]
- Egevad L, Algaba F, Berney DM, et al. Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch 2011;459:175-82. [PubMed]
- Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013;111:753-60. [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Kryvenko ON, Epstein JI. Changes in prostate cancer grading: Including a new patient-centric grading system. Prostate 2016;76:427-33. [PubMed]
- Moch H, Humphrey P, Ulbright T, et al, editors. WHO classification of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2016.
