The pathology of urinary bladder lesions with an inverted growth pattern
In addition to simultaneously occurring in multiple locations, urothelial tumor-like lesions and/or neoplastic lesions, particularly the latter, are characterized by another significant feature, that is, the diversity of growth patterns. These lesions can manifest as papillary (exophytic) as well as flat and inverted (endophytic) growth patterns. Most clinical cases of urothelial neoplasm present with a papillary growth one, which accounts for about 85% of urothelial neoplasms (1), while fewer cases present with flat or inverted growth patterns.
At present, there is much research on papillary urothelial neoplasm, especially noninvasive urothelial papillary neoplasm, owing to their frequent occurrence in clinical settings. In 2004, the World Health Organization and International Society of Urological Pathology (WHO/ISUP) conducted a detailed histological classification of these papillary urothelial neoplasms as follows: benign papilloma, papillary urothelial neoplasm of low malignant potential (PUNLMP), noninvasive papillary urothelial carcinoma (low grade or high grade), and invasive urothelial carcinoma (2). This grading system has the advantages of being intuitive, as well as having strong reproducibility and operability, which contributes to the standardization in the pathological diagnosis of noninvasive urothelial papillary neoplasms. It not only provides the foundation of treatment for clinicians, but also offers the patients the chance for a proper cure.
In contrast to papillary urothelial ones, urothelial neoplasms with flat and inverted growth patterns are relatively rare. Thus, few studies regarding these two types of urothelial neoplasms have been conducted worldwide, especially on neoplasms with an inverted growth pattern. Most pathologists believe that there is a strong similarity in the cellular morphology between flat and exophytic neoplasms (3-10). In 1998, the ISUP consensus first described and proposed a classification of flat neoplasms (11,12). In 2004, the WHO/ISUP proposed a rough grading system for flat neoplasms and indicated their morphological similarities to exophytic ones (2). As for lesions with an inverted growth pattern, although the ISUP consensus defined the inverted papilloma in 1998, its histological grading remained unaddressed (11,12). Despite awareness of this issue, the grading system proposed by the WHO/ISUP in 2004 failed to give any histological classification of urothelial neoplasms with an inverted growth pattern (2). This grading system only mentioned inverted papilloma and indicated that its inverted growth pattern might be confused with invasive carcinoma when found in an unusual location (2) (Table 1). In addition, certain sub-types of invasive urothelial carcinoma, such as the nested variant and large nested variant of urothelial carcinoma, cause great challenges and difficulties for pathologists to diagnose neoplasms with an inverted structure (13-18).
Apart from specific neoplastic lesions, some reactive or tumor-like lesions occurring on the urothelium also feature an inverted growth pattern, such as von Brunn nests (17,19-21), cystitis cystic/cystitis glandularis (20-25), and pseudocarcinomatous hyperplasia of the bladder (26-30). In some cases, these reactive lesions can be extremely easily confused with invasive carcinoma with an endophytic growth pattern, which increases the difficulty of diagnosing urothelial lesions with an inverted structure. Correct clinical guidance is based on correct pathological diagnosis, which generates proper treatment for the patient. Therefore, it is necessary for pathologists to be familiar with the diagnostic standards of these lesions. This paper presents a detailed and comprehensive introduction to neoplastic and tumor-like lesions with an inverted growth pattern from the perspective of their clinical, pathological, and few immunohistochemical features (Table 2).
Full table
Von Brunn nests
Von Brunn nests are usually observed in the normal urothelial mucosa, and they are believed to be caused by the invagination of normal urothelium in the lamina propria. It is reported that von Brunn nests can be seen in 89% of normal bladder mucosa (17,19). Careful examination of pathologic slides microscopically may show that these nest-like structures can be connected with the surface of the urothelium. This connection might sometimes be lost, and the nests will be distributed in an isolated fashion around the lamina propria, which may result in false images in different sections.
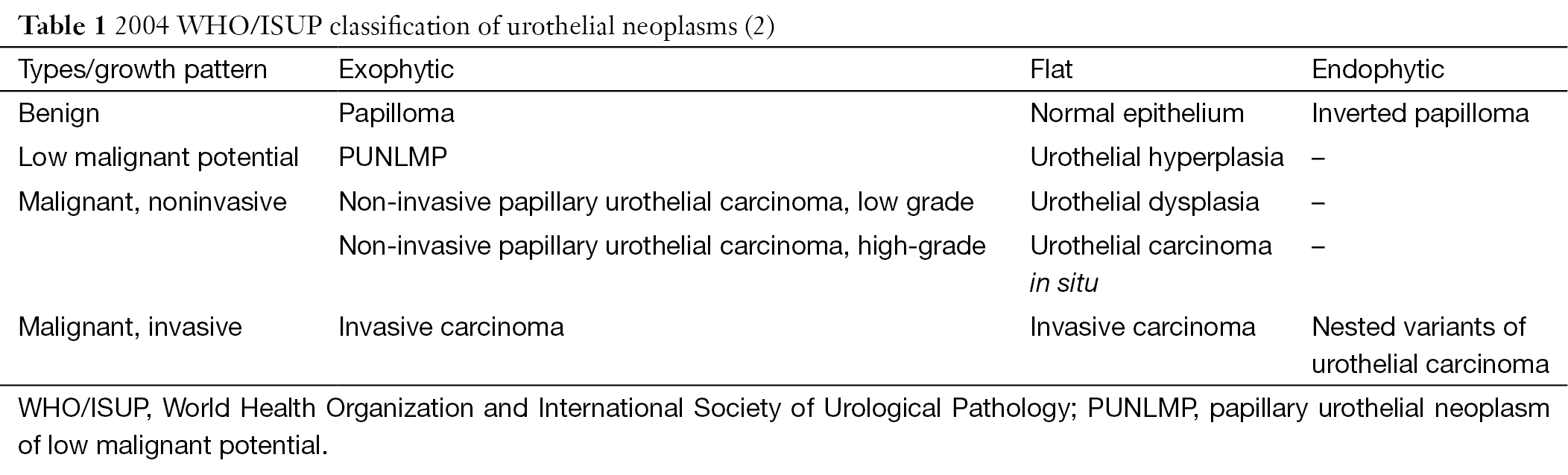
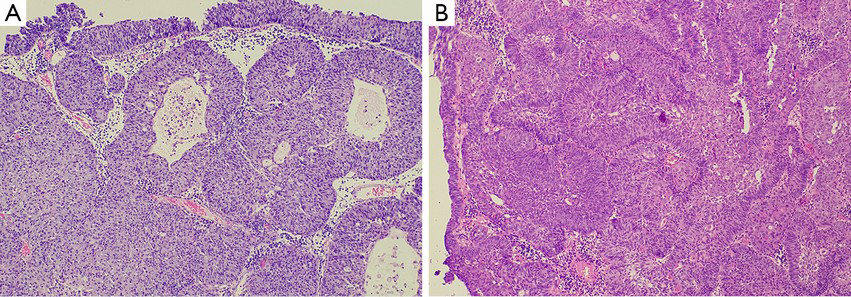
Although von Brunn nests are common and well known to pathologists, they haven’t given enough attention during routine pathology practice. In general, von Brunn nests are regular in shape, have smooth edges, are located on the superficial layer of the lamina propria, have relatively identical lesion depth, and lack nuclear atypia (17,19-21). But sometimes these lesions are vulnerable to being confused in morphology with nested variants of invasive urothelial carcinoma in the following two situations: first, if the von Brunn nests are so hyperplastic remarkably or florid that they can form significant solid cell nodules (Figure 1A); and second, if the von Brunn nests are located in the deep layer of the lamina propria and have an irregular basal layer of cells. In addition, remarkable hyperplasia may have a tumor-like appearance under cystoscopy, which will cause some difficulties in pathological diagnosis. Therefore, it is instrumental to be familiar with the histological features of von Brunn nests for its identification and the differential diagnosis between von Brunn nests and the nested variant of invasive urothelial carcinoma. The nested variant of invasive urothelial carcinoma can be disguised by a deceptively bland morphological appearance. But in reality, it is a highly invasive carcinoma with a high-grade of malignant potential. Thus, it is of great clinical importance to correctly distinguish these entities. Hyperplastic von Brunn nests tend to have a consistent depth during growth and are parallel to the surface of the epithelium. These lesions are evenly distributed in the lamina propria with regular shape, smooth-edged, and different sizes (17,19-21). Sometimes relatively large cell nests can be visible. The cells in von Brunn nests often lack nuclear atypia (17,19-21). The urothelium in the center of some cell nests may be lined with cystic or adenoid lacunae, which mimics the structure of cystitis cystica and cystitis glandularis (Figure 1A) (17,19-21). Usually von Brunn nests consist of loose connective tissue with regular gaps. Most cases of von Brunn nests show a symmetric growth pattern without lamina propria and muscular invasion. However, in the case of small biopsy specimens in which no lamina propria structure can be seen, there can be failure in the differential diagnosis based on the presence of invasion, even lamina propria invasion (17,19). In contrast, the capillary network surrounding the cell nests and the adjacent stroma resembling the normal mucosa can provide some clues for the differentiation between von Brunn nests and nested variant of invasive urothelial carcinoma. Generally, the diagnosis of malignancy is supported by the following evidence: the presence of crowded and irregular arrangements of nests with reticular network; the appearance of cellular atypia in the deep part of the lesions; and an accompanying invasive growth pattern. Results from current studies have shown that the application of immunohistochemical staining (including MIB-I, CK20, p27, and p53 protein staining) does not have a significant value in the differentiation between von Brunn nests and nested variant of invasive urothelial carcinoma (22).
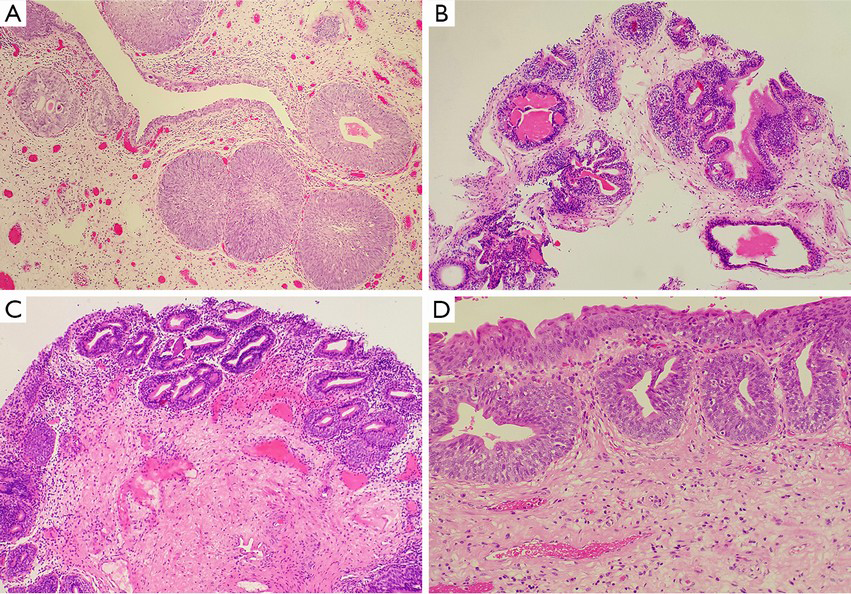
It should be emphasized that all the lesions occurring on the normal urothelium, including reactive proliferation, atypical hyperplasia, and carcinoma in situ, can involve von Brunn nests. Occasionally, nuclear atypia may be visible in von Brunn nests. This atypia is generally regarded as reactive atypical hyperplasia unless it is accompanied by atypical hyperplasia and carcinoma in situ at the same time.
Cystitis cystica and cystitis glandularis
Cystitis cystica is a group of urothelial disorders featuring cystic cavities in the center of the von Brunn nests and dilated cystic cavities lining the true urothelium (31). Cystitis glandularis is a term describing the glandular epithelium with mucosal cells lining the cystic cavity, with cuboidal or columnar epithelium in most cases (20-25). As with von Brunn nests, cystitis cystica and glandularis generally occur on the normal urothelial mucosa. Cystitis cystica and glandularis are observed in 60% of the normal bladder mucosa in autopsy cases (32). Cystitis cystica and glandularis usually occur concurrently with von Brunn nests, and all of them are regarded as a type of reactive lesion. Research has indicated that these lesions do not contribute to the risk factors of bladder cancer, and there is no relationship between the appearance of this lesion in the bladder and the occurrence of bladder cancer (31). Cystitis cystica and glandularis show no significant epidemiologic difference considering patient sex (20-25). They are mainly seen in adults but also can occur in children. Occasionally cystitis glandularis may be accompanied by intestinal metaplasia, which is seen by mucus columnar epithelium and goblet cells (32). Furthermore, when the immunophenotype is similar to the mucous epithelium of the intestinal type (CK20+, CDX2+), it can be diagnosed as intestinal-type cystitis glandularis. But Paneth cells can be seen rarely (32). In cases of cystitis glandularis in which the intestinal metaplasia is the entire or primary manifestation, the term “intestinal metaplasia” is commonly used as a replacement for the diagnosis of “intestinal-type cystitis glandularis” (32).
In most cases, it is relatively easy to diagnose cystitis cystica and cystitis glandularis pathologically. However, in those cases with significant hyperplasia, it is necessary to differentiate them from invasive carcinoma accompanied by adenoid structures, which include invasive urothelial carcinoma associated with glandular differentiation, microcystic variant of invasive carcinoma, and invasive adenocarcinoma (10,31,32). The above-mentioned features for differentiation between von Brunn nests and invasive carcinoma are still applicable in thess cases (Figure 1) (31). In addition, the interstitial morphology sometimes contributes to the differentiation between reactive lesions and invasive carcinoma, for example, the occurrence of desmoplastic reactions supports the presence of invasive carcinoma (31). Furthermore, great attention should be paid to cases in which there is mucus overflow from the glandular structure of intestinal-type cystitis glandularis which should be differentiate from mucinous adenocarcinoma. Although the differential diagnosis is difficult in these cases, it is helpful to have a clear understanding of the variants of cell morphology, the deficiency of free-floating epithelial nests in the mucus lake, and the lack of muscularis propria involvement.
Research has shown that significant telomere shortening exists in urothelial lesions associated with intestinal metaplasia with/without cystitis glandularis (24), which increases the possibility that intestinal metaplasia is the precancerous lesion of adenocarcinoma. However, most long-term fellow-up, clinical, and pathological research have found that urothelial lesions with intestinal metaplasia failed to conclusively increase the occurrence of carcinoma (22). Therefore, further studies are needed to discover the association between urothelial lesions and intestinal metaplasia and carcinoma.
Pseudocarcinomatous hyperplasia
Pseudocarcinomatous hyperplasia is a rare reactive hyperplasia on the epithelium of the bladder mucosa that is commonly seen after radiotherapy and chemotherapy, or can be the result of hemorrhage/irritation (10,26-32). Patients who have received radiotherapy or chemotherapy in or around their bladder usually suffer from hematuria within the next 0–7 years (average time: 2 years) (27). Pseudocarcinomatous hyperplasia may also mimic invasive carcinoma through the presence of abnormal changes in histological structure and cellular morphology. Pseudocarcinomatous lesions always consist of irregular cell nests with an invasive appearance. Occasionally, they are synchronous with squamous metaplasia. Their nuclei are mildly to moderately multi-morphological and usually show no mitotic figures, although some active mitotic figures might be visible (>8–10 high power field) (27). When clearly observed, Pseudocarcinomatous lesions are usually limited to the mucosa. Additionally, some diagnostic clues can be obtained on the basis of the other symptoms, including ulcers, inflammation, hemorrhage, congestion of blood vessels, fibrin deposition, and edema. The following indications can also help pathologists to exclude invasive carcinoma: a definite history of clinical treatment (27), the invasive cell nests surrounding dilated small blood vessel with fibrinous thrombus formation (26), and the absence of carcinoma in situ transformation on the urothelium far from the lesion (32). It is important to remember that radiotherapy and chemotherapy are risk factors for carcinoma, especially with cyclophosphamide treatment (32-37). Thus, if the diagnosis is questionable, careful histological examination should be conducted to exclude the occurrence of carcinoma after treatment. Furthermore, the evaluation of patients with a history of bladder cancer should be performed with caution.
Pseudocarcinomatous hyperplasia may occasionally be observed in patients without a history of radiotherapy or chemotherapy (29,30). Predisposing factors include causes of hemorrhage or irritation, such as chronic catheterization (29,30). In these cases, the pathologic morphology can demonstrate proliferation resembling that after radiotherapy or chemotherapy, and the definitive diagnosis can be obtained from the presence of peripheral inflammation, edema, hemorrhage, dilated vessels, fibrin exudation, and hemosiderin deposition. Interestingly, the proliferated cells in this case usually tend to have an eosinophilic cytoplasm (29,30), which can be misdiagnosed as abnormal or squamous metaplasia of invasive urothelial carcinoma. However, careful observation reveals that the lesions do not involve the muscularis propria and lack significant nuclei atypia despite an invasive appearance. It remains unclear whether the above-mentioned features are precancerous lesions, and thus, further investigations are needed. However, a definite answer may never be obtained owing to the rarity of this disease.
Inverted papilloma
Inverted papilloma of the bladder (IPB) is a rare benign tumor in the bladder. Its morbidity accounts for 1.4% to 2.2% of all urothelial neoplasms (9,38). The term “inverted papilloma” was first introduced in the study by Potts et al. in 1963 (39). IPB is most commonly diagnosed in elderly people, aged 60–70 years, especially in men (the ratio of morbidity in men to women is 7.3:1) (10). At present, the cause of IPB remains unknown, although it has been proposed that IPB is related to smoking, chronic infection of the urinary bladder, hyperplasia of von Brunn nests, and urinary tract obstruction (9,38,40-42). IPB may occur at any site of the urinary tract, but is primarily present in the bladder. Clinically, patients with IPB present with symptoms similar to those of transitional cell carcinoma of the bladder, exhibiting intermittent and painless macroscopic hematuria accompanied by symptoms of urothelial irritation in some cases. Patients with IPB with a large neoplasm may suffer from dysuria (9,38,41,42). Occasionally, asymptomatic IPB may be detected during physical examination. Although IPB can occur on any site of the bladder, it mainly occurs in the neck, trigone, and sidewall of the bladder. But it is rarely seen in the top of the bladder. Under cystoscopy, IPB presents primarily as a pedunculated (such as papillary, polypoid and seaweed-like) or sessile mass with a relatively obvious smooth surface. The diameter of the mass is less than 3 cm in most cases but can sometimes be much larger, with diameter of up to 8 cm (9,38,41,42). Generally, the neoplasms are single lesions, though some cases may be multiple lesions (1.3–4.4%) (38,43,44).
Microscopically, the surface of the IPB is lined with a normal or thin layer of urothelium. The neoplasm grows endophytically from the epithelial mucosa to the lamina propria, which forms different-sized epithelial nests. Some cell nests are interconnected to form the trabecular type (Figure 2A), with peripheral basal cells in a columnar and palisade arrangement, vertical to the basal lamina (Figure 2B). An increased thickness of the basal lamina connected closely with the basal cell is usually observed. Cells in the center of the nest, generally characterized by their thin-spindled appearance and serpiginous configuration, are arranged in parallel with the basal lamina (Figure 2B). Mitotic figures are rarely seen (9,38,41,42,45). In the center of the nest, micro cystic or adenoid structure formed. The morphology of the cells in the innermost layer resembles typical surface “umbrella” cells of the urothelium (Figure 2C). Pathologically, IPB can be classified into three subtypes: the trabecular subtype, the adenoid subtype, and the mixed one (46). The trabecular type, which is the classic type of IPB, is thought to originate from the basal cells. The trabecular structure is relatively thin with consistent thickness, has a regular arrangement in the basal part, and originates from the same depth (10,46). Additionally, the stroma within the trabecular structure is mainly loose connective tissue (Figure 2D), though fibrosis may occur in some cases. In some cases, the epithelium on the surface of the lesions is accompanied by exophytic growth (47,48). The presence of focal squamous metaplasia or neuroendocrine differentiation is rarely observed. Significant cytological atypia of the neoplastic cell nuclei is almost absent under close observation (48).
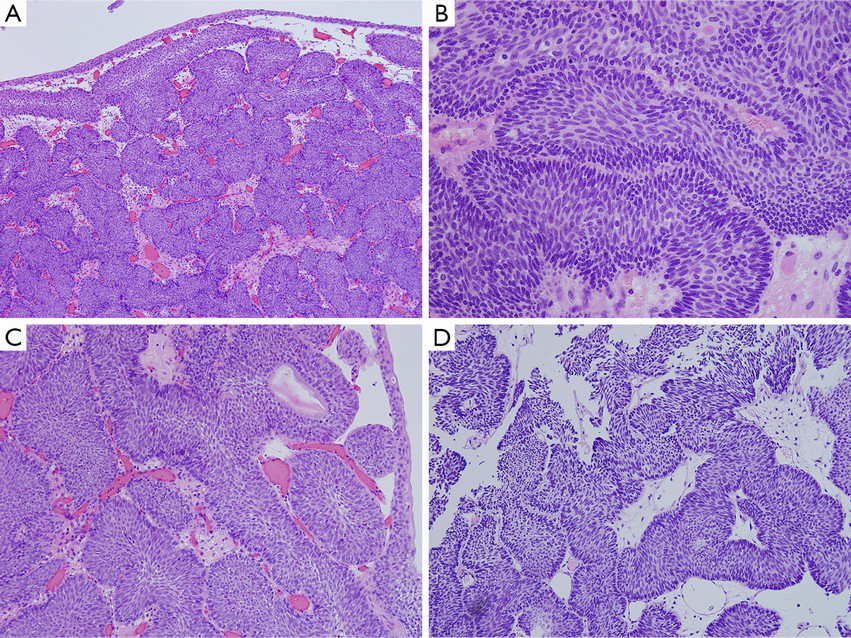
The glandular subtype of IPB has overlapping morphological features with cystitis glandularis/cystic. And it is believed the glandular subtype of IPB originate from the extension of cystitis glandularis/cystica (10,49). Microscopic observation shows that the glandular subtype of IPB consists of nests with either pseudo-glandular spaces lined by urothelium or true glandular spaces containing goblet cells. The cytoplasm of the tumor cells in some cases has a vacuolated or foamy appearance, which can be misdiagnosed as urothelial carcinoma.
As for the pathological diagnosis of IPB, Amin et al. proposed nine strict diagnostic criteria (6): (I) inverted growth pattern; (II) smooth surface lined with mature urothelium; (III) uniform epithelial morphology; (IV) tumor cells with consistent arrangement; (V) no or few mitotic figures; (VI) possible presence of microcyst formation; (VII) possible presence of non-keratinizing squamous metaplasia; (VIII) no exophytic component; and (IX) noninvasive. In fact, IPB, if it was diagnosed according to the above criterias strictly in a clinical setting, has a benign process with a recurrence rate of 1%, and can be cured with secondary complete excision, just like von Brunn nests (47,50). For the treatment of IPB, transurethral resection of the bladder tumor (TURBT) is recommended, which requires the excision of the lamina propria or superficial muscularis propria invasion, and partial excision of the bladder may be necessary for relatively large neoplasms (47,50-53).
Noninvasive inverted urothelial neoplasm
According to the 2004 WHO/ISUP definition, inverted papilloma has benign biological behavior and process, almost without recurrence, extension, or metastasis after complete surgical excision. In addition, no further treatment is needed after excision, even intravesical instillation (47,50-53). However, numerous doubts on the benign biological behavior of IPB have been raised in recent years (44,48,54,55). An increasing number of pathologists have been faced with confusion during the pathological diagnosis of this condition. For example, some cases have an extremely similar structure to IPB at low magnification but have relatively large cords and nests of neoplastic cells with different sizes and irregular morphology. Furthermore, the nuclei of these neoplastic cells display a certain degree of atypia, and the upward movement of nucleic mitotic figures may be present. There may even be formation of solid structures in these cases, and fibrovascular cores may be observed in the center of some cellular nests. On the other hand, urologists have also found that 3–5% of IPB cases that are diagnosed pathologically have characteristics of malignant neoplasms, which are susceptible to recurrence, extension, and metastasis (51,52). It is difficult to explain this phenomenon thoroughly with benign neoplasms. Therefore, there have been continuous reports indicating that IPB may have malignant potential and is associated with urothelial carcinoma to a certain degree. IPB has drawn attention owing to its increased malignancy, multi-occurrence, and recurrence. As mentioned above, if a pathological diagnosis is obtained according to strict definitions and criteria, IPBs, mimicking a von Brunn nests, have a benign process with a recurrence rate of 1% and can be cured with a complete secondary excision. The main reason for regarding IPB as having a low-grade malignant potential or malignant biological behavior with a recurrence rate as high as 5% is inaccurate diagnostic criteria and insufficient knowledge on urothelial neoplasms with an inverted growth structure (6,47,50).
Since the WHO/ISUP consensus in 1998, experts have recognized that inverted neoplasms are a continual challenge in the pathological diagnosis of urologic diseases (11-12). Unfortunately, the 2004 WHO/ISUP grading system did not provide explanations of noninvasive, inverted, and dysplastic urothelial neoplasms (2). Because of the lack of guidance, the diagnosis of such neoplasms is not clear. For example, noninvasive inverted non-benign neoplasms has been named as “atypical inverted papilloma”, “borderline inverted urothelial neoplasm of low-grade malignant potential”, “dysplastic noninvasive inverted urothelial carcinoma”, and “noninvasive inverted urothelial carcinoma”, or other names (7-10,44,47,48,50-61). Owing to the lack of standardized terminologies for the pathological diagnosis, clinicians are often at a loss, which subsequently leads to relatively inconsistent treatments. This is evident in how some patients receive intravesical instillation, whereas some do not. As a result, it becomes a difficult problem for the clinicians and pathologists to diagnose and treat these kind of noninvasive urothelial neoplasms with a certain degree of atypia in its histological structure and cellular morphology.
In 2012, experts from the International Consultation on Urological Diseases (ICUD) concluded that it is urgent and necessary to establish a histological grading system for inverted urothelial neoplasms (5,57). In particular, the current grading system was not suitable for cases with an inverted structure. Furthermore, the ICUD pointed out that a lack of awareness and guidance for inverted urothelial neoplasms has led to some neoplasms, especially those with inverted PUNLMP being assigned different names in literature and reports. Consequently, the ICUD recommended that the WHO/ISUP system should establish a grading standard and apply this standard for the grading of inverted lesions, including inverted papilloma, inverted PUNLMP, low-grade inverted urothelial carcinoma, and high-grade inverted urothelial carcinoma (Table 3) (5,57). This made the current histological classification of flat and exophytic papilloma applicable to inverted lesions. Although part of the data indicates that it is feasible and effective to apply the histological grading systems proposed by the WHO/ISUP to endophytic lesions, especially PUNLMP, the ICUD suggests that it is still important to conduct comprehensive communication with clinicians, because the data collections of these kind of neoplasms have just begun. Clinicians should be aware of the limited prospective implications of this diagnosis. However, the application of standardized diagnostic criteria and terminologies will contribute to a better understanding and allow further in-depth studies on neoplasms. In daily clinical practice, neoplastic cases with mixed growth of both exophytic and entophytic neoplasms are commonly observed. Therefore, the ICUD recommended that this diagnostic term should only be used in those cases in which the inverted lesions are the prominent lesions.
Full table
In 2014, the ICUD experts proposed the above-mentioned classification. However these suggestions, which can be seen in Table 3, only consider atypical cellular morphology. However, routine clinical work has shown that some other features have the same significance as cellular atypia in the grading of exophytic or flat urothelial neoplasms, such as the regularity and uniformity of the entophytic trabecular structure, thickness of cellular layers, existence of central “umbrella” cells, presence of mitotic figures, layers with appearance of mitotic figures, and presence of pathological mitotic figures. So we suggest we should take into account these features in the grading of inverted neoplasms (Table 4). The following situations within the trabecular structure indicate the occurrence of noninvasive urothelial carcinoma: the presence of an irregular and/or inconsistent trabecular structure; a significant increase in cellular layers; the absence of central “umbrella” cells; and the presence of mitotic figures or mitotic figures moving from the outer layers toward the center, especially the presence of pathological mitotic figures. Furthermore, the appearance of exophytic papillary structure in neoplasms is of great value for the evaluating and classifying inverted neoplasms. In general, it is rare to observe exophytic papillary urothelium in inverted papilloma, whereas it is common to observe some exophytic papillary urothelium in noninvasive inverted low-grade malignant potential or malignant neoplasms. In addition, cystoscopy might also reveal the following indicators: inverted papilloma is typically characterized as a single peduncle mass with smooth surface; but noninvasive inverted urothelial carcinoma is typically characterized as multiple masses with a wide base, cauliflower-like appearance, and a relatively jagged surface.
Full table
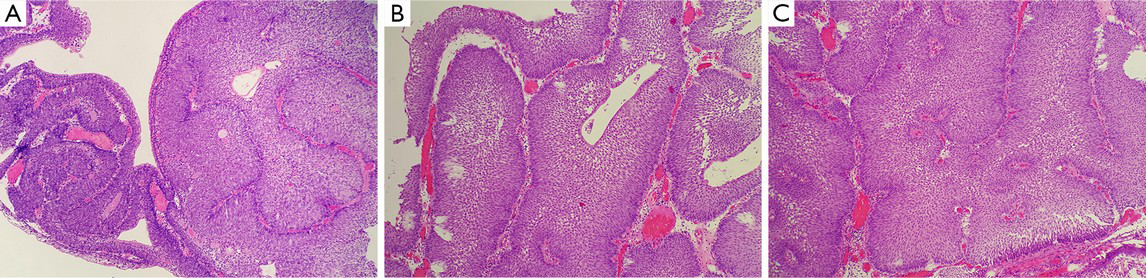
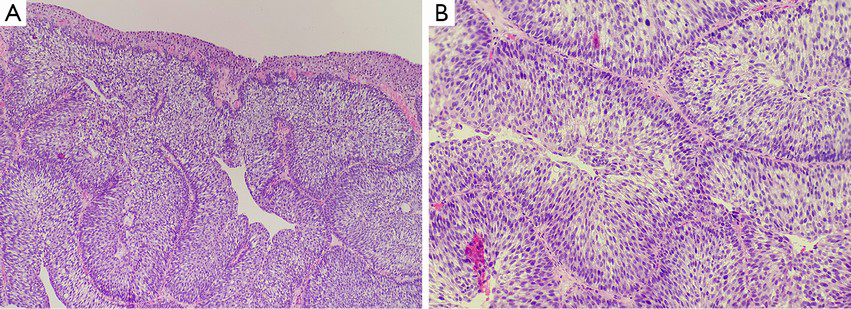
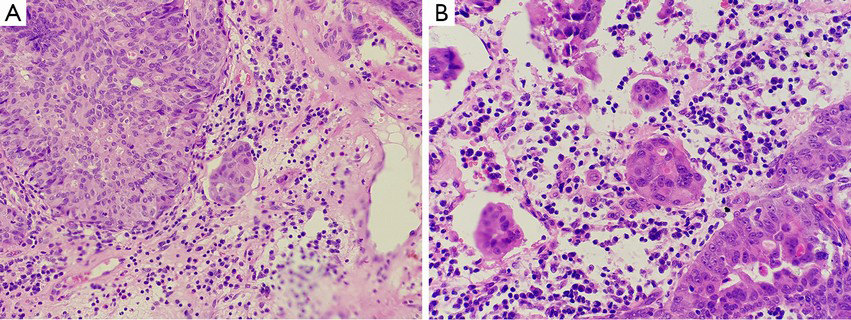
Inverted papilloma and noninvasive urothelial carcinoma with an inverted growth pattern of the bladder may be difficult to distinguish histologically, especially in cystoscopy-obtained biopsies. The neoplastic tissues are small, obscure and unclear in structure and cellular morphology because of deformation. Thus, pathologists hope some assistance in the grading and confirmation process by using other features, such as immunohistochemical stains and molecular genetics examination. CK20 and Ki67 are the indicators for the differentiation and proliferation of urothelial cells that reflect the changes in differentiation and proliferation of neoplasms with an inverted growth pattern. The expression of CK20 in normal urothelial cells is limited to the superficial cap cells, but it is not observed in abnormal proliferation, when its expression can be seen in the entire cellular layer. Some studies suggested that CK20 stain is of great significance in distinguishing between IPB and noninvasive urothelial carcinoma with an inverted growth pattern (51). The fact that urothelial carcinoma with an inverted growth pattern stains positively for CK20 in the entire layer contributes to its pathological diagnosis. However, an increasing number of reports have indicated that further studies are needed for the value of CK20 in distinguishing IPB and noninvasive urothelial carcinoma with an inverted growth pattern (52). In addition, Ki-67 antigen are a reliable indicator in reflecting the activity of cellular proliferation. Generally, in cases of true inverted papilloma, cellular activity is very low, the Ki67 index is usually less than 1%, and positive cells are observed only in the basal layer of the epithelium. As the grade of histological lesions increases, the Ki67 index increases gradually, and the positive cells gradually extend to the center. This feature is of great importance for the correct evaluation of the histological grading of neoplasms (7,51,52,62). In addition, some authors believed that p53 essentially plays no role in the differentiation between inverted papilloma and inverted urothelial carcinoma of low-grade of malignant potential (51). The significance of the expression of p53 in some inverted papilloma needs to be further studied. UroVysion FISH was originally used for urine cytology, as an aid in the initial diagnosis of bladder carcinoma in patients with hematuria and in the detection of recurrence after surgery in patients previously diagnosed with bladder cancer (63). It is designed to detect the benign or malignant feature of cells by marking chromosomes 3, 7, 17, and 9p21 in an individual cell and observing an amplification of 3, 7, 17, and deletion of 9p21. Some studies have found that 79% of cases with inverted urothelial carcinoma show abnormalities in chromosomes 3, 7, 17, and 9p21 (51,62). In contrast, only a few cases with inverted papilloma present abnormalities of single chromosomes. This indicates that UroVysion FISH may be valuable in the differentiation between inverted papilloma and noninvasive urothelial neoplasm with an inverted growth pattern. However, due to the limited number of reports, further studies of this are needed.
Inverted urothelial carcinoma
Inverted urothelial carcinoma is a rare subtype that was first described by Amin et al. (6). Subsequently, there were also corresponding reports of inverted urothelial carcinoma, but so far the reported cases was less than 40 (7,8,51,64-66). Most of the reported cases were noninvasive inverted ones, and few of them were invasive inverted urothelial carcinoma. To non-invasive neoplasms, it is necessary to distinguish them from inverted papilloma. Just as stated previously, the difficulties may reduce along with recognizing and being familiar with the distinguishing features of each type. However, determining the invasive features of inverted urothelial carcinoma remains a challenge. Even for exophytic and flat urothelial neoplasms, the basal part of these neoplastic tissues has a pushing growth pattern towards the lamina propria (2), thus, it is difficult to distinguish a single pushing growth pattern and expansive infiltration. In addition, the differential diagnosis becomes more difficult because inverted urothelial carcinoma also exhibits endophytic growth in the lamina propria. Therefore, it is unrealistic to determine whether urothelial carcinoma is accompanied by invasive expansive infiltration.
Theoretically, the smoothness and/or completeness of the basement membrane in the urothelium are key factors in determining whether the neoplasm is invasive. The invasion of the neoplasm should be considered when there are irregular cellular nests and damage or absence of the basement membrane. Unfortunately, this differentiation method is difficult to perform and lacks repeatability, hence has reduced practical value. As a result, we recommend applying the criteria for invasive urothelial papilloma to the diagnosis of inverted urothelial carcinoma. Micro-nested, cluster-like, or individual/cords of the tumor cells indicate an early stage of invasion (Figure 6). Other histological standards also contribute to the diagnosis of invasive lamina propria, including a stromal reaction of pro-fibrogenic connective tissue, presence of tumor cells in the constriction gap, and the presence of abnormal differentiation of the tumor cells. A definitive diagnosis of invasive lesions can also be obtained from other evidence, such as a significant inflammation reaction in the lamina propria. Attention should also be paid to micro-invasive lesions, where the stromal reaction is absent. This causes greater difficulty in identifying invasive lesions by simple HE morphology. In this case, immunohistochemical cytokeratin staining will help in the differential diagnosis. Clinically invasive urothelial carcinoma with an inverted growth pattern is characterized by highly aggressive biological behavior, is susceptible to recurrence, and often progresses and metastasizes. Thus, treatment and care should be provided after surgery.
Nested variant of invasive urothelial carcinoma
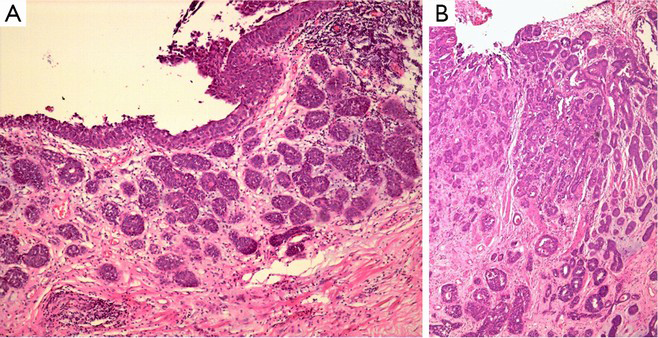
Nested variant of urothelial carcinoma is one of the variants of urothelial carcinoma that was first reported by Stern (67) and added to the WHO classification in 2004 (2). Less than 50 cases have been reported so far (5). The epidemiology and age of onset are similar to the classic urothelial carcinoma, occur more frequently in men than in women (5,67). Clinically, nested variant of urothelial carcinoma are invasive neoplasms and 70% of the patients died within 4–40 months after the diagnosis (5). But it is characterized by an unusual, bland morphology which mimics some benign urinary bladder lesions, even von Brunn nests (Figure 7A). So, morphologically, nested variant of urothelial carcinoma is usually described as a “pseudo-benign” neoplasm (5,13-15,67). The following features are commonly observed in the deeper part of the neoplasm: the presence of tubular lumen, small or indistinct nuclei without nuclear atypia, and the consistent presence of focal and definite anaplastic cells manifesting as increasing nuclear size and thickening chromatin. The following features contribute to the diagnosis of malignant lesions: cells in the deeper part of the lesions are increasingly anaplastic and invasive, usually invading the muscularis layers (Figure 7B) (5,13-15,67). The differential diagnosis should be conducted to distinguish this from florid von Brunn nests, cystitis cystica and cystitis glandularis, inverted papilloma, nephrogenic metaplasia, carcinoid, and paraganglioma. Deep infiltration is the most effective diagnostic method to distinguish carcinoma from benign hyperplasia, and the diagnosis of carcinoma can be supported by the occasional presence of nuclei atypia and intensive and/or irregular distribution of small cells (5,13-15,67). Typical nephrogenic metaplasia includes mixed-morphology of tubular, papillary, and other components, whereas deep infiltration of the muscular layer is rare in this case. Nested variants may be similar to paraganglioma, although the latter has obvious vascular networks, and this phenomenon is rarely if ever observed in nested variants of invasive urothelial carcinoma.
Large nested variant of urothelial carcinoma
Similar to the small nested variant of urothelial carcinoma, the large nested variant of urothelial carcinoma is a variant of urothelial carcinoma with a “pseudo-benign” appearance (16). It is well known that some benign urothelial lesions, such as von Brunn nests, are easily misdiagnosed as urothelial carcinoma (17). Similarly, it is of great significance to be familiar with the large nested variant of urothelial carcinoma, as it usually gives clinicians the illusion of being a benign urothelial neoplasm but does in fact invade the deep tissue of the bladder wall (Figure 8A). The large nested variant of urothelial carcinoma features morphologically moderate neoplastic cells arranged in a large nested structure with wide and expanding invasive growth patterns, which resemble verrucous carcinoma (Figure 8B). Compared with the small nested variant of urothelial carcinoma, the large variant shows low-grade morphological transformation on the surface of the neoplasm (5). In the serial case report conducted by Cox et al., clinical follow-up was available for 17 of 23 patients, and 6 of these 17 cases presented with continuous or progressive neoplasms (16). This indicates that the low-grade appearance on the surface of the neoplasm can be very deceptive.
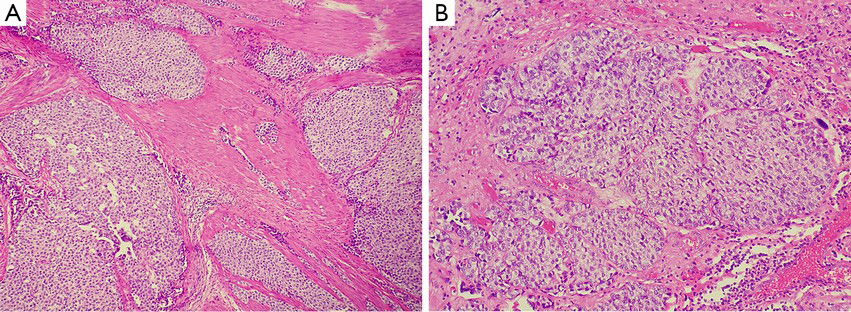
Large-scale and in-depth cohort studies are needed in order to better identify this variant, which has significant implications to the understanding of its epidemiological characteristics and prognostic features. At present, certain characteristics can help us to differentiate the large variant of urothelial carcinoma from other similar lesions. First, compared to the “garden variety” of invasive urothelial carcinoma, this variant has a milder morphological appearance. But it doesn’t have a similar atypia to that of urothelial carcinoma of low-grade. Even during reactive hyperplasia, its atypia usually exceeds that of von Brunn nests. Second, in the small nested variant of urothelial carcinoma, the degree of nuclear atypia usually exceeds that of the larger variant. Diagnostic features supporting the large nested variant of urothelial carcinoma include the haphazard and irregular distribution of nested structures on the inner wall of the bladder. Third, sometimes this variant might be mistaken as residual urachus, mesonephric ducts, or paramesonephric ducts. Infiltration of the bladder wall by tumor tissues, especially the deeper muscle layers, has extremely significant implications. This is because non-invasive endophytic urothelial carcinoma will not exhibit infiltration to large caliber bundles of muscularis propria. Last, attention should be paid to the normal infiltration of the lesion, as clinical presentations in one-third of previous reports can help us to confirm the presence of infiltration (5).
In conclusion, although rare, neoplasms with an inverted growth pattern or tumor-like lesions in the urinary bladder have a very wide histo-morphological range, including reactive/tumor-like lesions, benign neoplasms, neoplasms of low-grade malignant potential, noninvasive urothelial carcinoma of low-grade, noninvasive inverted urothelial carcinoma of high-grade, invasive urothelial carcinoma of high-grade, and other histological variants of invasive urothelial carcinoma with endophytic growth patterns. Different lesions have different biological behaviors, and thus, corresponding clinical treatment should be applied. Therefore, it is helpful for pathologists and clinicians to be familiar with and understand these lesions in order to provide patients with a proper diagnosis and subsequent treatment, and then increasing quality of life as well as survival rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol 2004;22:86-92. [PubMed]
- Eble JN, Sauter G, Epstein JI et al. eds. WHO Classification of tumors. Pathology & Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004;89-123.
- Wang C, Maxwell JP, Yilmaz A, et al. Primary papillary urothelial neoplasm of low malignant potential (PUNLMP) including PUNLMP with inverted growth: outcome analysis. Mod Pathol 2012;25:226A.
- Akki AS, Richards K, Smith ND, et al. Inverted/endophytic growth pattern of papillary urothelial neoplasms of the bladder: clinicopathological study in 225 transurethral resection specimens. Mod Pathol 2014;27:212A.
- Amin MB, Smith SC, Reuter VE, et al. Update for the practicing pathologist: The international consultation on urologic disease-European association of urology consultation on bladder cancer. Mod Pathol 2015;28:612-30. [PubMed]
- Amin MB, Gómez JA, Young RH. Urothelial transitional cell carcinoma with endophytic growth patterns: a discussion of patterns of invasion and problems associated with assessment of invasion in 18 cases. Am J Surg Pathol 1997;21:1057-68. [PubMed]
- Jones TD, Zhang S, Lopez-Beltran A, et al. Urothelial carcinoma with an inverted growth pattern can be distinguished from inverted papilloma by fluorescence in situ hybridization, immunohistochemistry, and morphologic analysis. Am J Surg Pathol 2007;31:1861-7. [PubMed]
- Terada T. Inverted variant of urothelial carcinoma of the urinary bladder: a report of three cases and a proposal for a new clinicopathologic entity. Int J Clin Exp Pathol 2013;6:766-70. [PubMed]
- Sung MT, Maclennan GT, Lopez-Beltran A, et al. Natural history of urothelial inverted papilloma. Cancer 2006;107:2622-7. [PubMed]
- Hodges KB, Lopez-Beltran A, Maclennan GT, et al. Urothelial lesions with inverted growth patterns: histogenesis, molecular genetic findings, differential diagnosis and clinical management. BJU Int 2011;107:532-7. [PubMed]
- Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22:1435-48. [PubMed]
- Mostofi FK, Davis CJ, Sesterhenn IA. World Health Organization International Histological Classification of Tumours. Histological Typing of Urinary Bladder Tumours, 2nd edtion. Springer Verlag: Berlin, Heidelberg, 1999;3-23.
- Wasco MJ, Daignault S, Bradley D, et al. Nested variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of 30 pure and mixed cases. Hum Pathol 2010;41:163-71. [PubMed]
- Venyo AK. Nested variant of urothelial carcinoma. Adv Urol 2014;2014:192720.
- Dhall D, Al-Ahmadie H, Olgac S. Nested variant of urothelial carcinoma. Arch Pathol Lab Med 2007;131:1725-7. [PubMed]
- Cox R, Epstein JI. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of noninvasive papillary urothelial carcinoma. Am J Surg Pathol 2011;35:1337-42. [PubMed]
- Volmar KE, Chan TY, De Marzo AM, et al. Florid von Brunn nests mimicking urothelial carcinoma: a morphologic and immunohistochemical comparison to the nested variant of urothelial carcinoma. Am J Surg Pathol 2003;27:1243-52. [PubMed]
- Toll AD, Epstein JI. Invasive low-grade papillary urothelial carcinoma: a clinicopathologic analysis of 41 cases. Am J Surg Pathol 2012;36:1081-6. [PubMed]
- Wiener DP, Koss LG, Sablay B, et al. The prevalence and significance of Brunn's nests, cystitis cystica and squamous metaplasia in normal bladders. J Urol 1979;122:317-21. [PubMed]
- Cheng L, Bostwick DG. Overdiagnosis of bladder carcinoma. Anal Quant Cytol Histol 2008;30:261-4. [PubMed]
- Young RH. Non-neoplastic disorders of the urinary bladder. In: Bostwick DG, Cheng L, eds. Urologic Surgical Pathology, 2nd ed. Philadelphia: Mosby Elsevier, 2008:215-56.
- Smith AK, Hansel DE, Jones JS. Role of cystitis cystica et glandularis and intestinal metaplasia in development of bladder carcinoma. Urology 2008;71:915-8. [PubMed]
- Aabech HS, Lien EN. Cystitis cystica in childhood: clinical findings and treatment procedures. Acta Paediatr Scand 1982;71:247-52. [PubMed]
- Morton MJ, Zhang S, Lopez-Beltran A, et al. Telomere shortening and chromosomal abnormalities in intestinal metaplasia of the urinary bladder. Clin Cancer Res 2007;13:6232-6. [PubMed]
- Talmon GA, Khan A, Koerber R, et al. A cribriform urothelial neoplasm of the renal pelvis: an adenoid cysticlike variant of inverted urothelial papilloma or florid ureteritis cystica? Arch Pathol Lab Med 2010;134:1557-9. [PubMed]
- Baker PM, Young RH. Radiation-induced pseudocarcinomatous proliferations of the urinary bladder: a report of 4 cases. Hum Pathol 2000;31:678-83. [PubMed]
- Chan TY, Epstein JI. Radiation or chemotherapy cystitis with "pseudocarcinomatous" features. Am J Surg Pathol 2004;28:909-13. [PubMed]
- Crew JP, Jephcott CR, Reynard JM. Radiation-induced haemorrhagic cystitis. Eur Urol 2001;40:111-23. [PubMed]
- Lane Z, Epstein JI. Pseudocarcinomatous epithelial hyperplasia in the bladder unassociated with prior irradiation or chemotherapy. Am J Surg Pathol 2008;32:92-7. [PubMed]
- Kryvenko ON, Epstein JI. Pseudocarcinomatous urothelial hyperplasia of the bladder: clinical findings and followup of 70 patients. J Urol 2013;189:2083-6. [PubMed]
- Hameed O, Humphrey PA. Pseudoneoplastic mimics of prostate and bladder carcinomas. Arch Pathol Lab Med 2010;134:427-43. [PubMed]
- Harik LR, O'Toole KM. Nonneoplastic lesions of the prostate and bladder. Arch Pathol Lab Med 2012;136:721-34. [PubMed]
- Bostrom PJ, Soloway MS, Manoharan M, et al. Bladder cancer after radiotherapy for prostate cancer: detailed analysis of pathological features and outcome after radical cystectomy. J Urol 2008;179:91-5; discussion 95. [PubMed]
- Liauw SL, Sylvester JE, Morris CG, et al. Second malignancies after prostate brachytherapy: incidence of bladder and colorectal cancers in patients with 15 years of potential follow-up. Int J Radiat Oncol Biol Phys 2006;66:669-73. [PubMed]
- Shah SK, Lui PD, Baldwin DD, et al. Urothelial carcinoma after external beam radiation therapy for prostate cancer. J Urol 2006;175:2063-6. [PubMed]
- Moon K, Stukenborg GJ, Keim J, et al. Cancer incidence after localized therapy for prostate cancer. Cancer 2006;107:991-8. [PubMed]
- Müller AC, Ganswindt U, Bamberg M, et al. Risk of second malignancies after prostate irradiation? Strahlenther Onkol 2007;183:605-9. [PubMed]
- Brown AL, Cohen RJ. Inverted papilloma of the urinary tract. BJU Int 2011;107 Suppl 3:24-6. [PubMed]
- Potts IF, Hirst E. Inverted papilloma of the bladder. J Urol 1963;90:175-9. [PubMed]
- Albores-Saavedra J, Chable-Montero F, Hernández-Rodríguez OX, et al. Inverted urothelial papilloma of the urinary bladder with focal papillary pattern: a previously undescribed feature. Ann Diagn Pathol 2009;13:158-61. [PubMed]
- Darras J, Inderadjaja N, Vossaert P. Synchronous inverted papilloma of bladder and renal pelvis. Urology 2005;65:798. [PubMed]
- Picozzi S, Casellato S, Bozzini G, et al. Inverted papilloma of the bladder: a review and an analysis of the recent literature of 365 patients. Urol Oncol 2013;31:1584-90. [PubMed]
- Rozanski TA. Inverted papilloma: an unusual recurrent, multiple and multifocal lesion. J Urol 1996;155:1391. [PubMed]
- Cheng CW, Chan LW, Chan CK, et al. Is surveillance necessary for inverted papilloma in the urinary bladder and urethra? ANZ J Surg 2005;75:213-7. [PubMed]
- Henderson DW, Allen PW, Bourne AJ. Inverted urinary papilloma: report of five cases and review of the literature. Virchows Arch A Pathol Anat Histol 1975;366:177-86. [PubMed]
- Kunze E, Schauer A, Schmitt M. Histology and histogenesis of two different types of inverted urothelial papillomas. Cancer 1983;51:348-58. [PubMed]
- Patel P, Reikie BA, Maxwell JP, et al. Long-term clinical outcome of inverted urothelial papilloma including cases with focal papillary pattern: is continuous surveillance necessary? Urology 2013;82:857-60. [PubMed]
- Broussard JN, Tan PH, Epstein JI. Atypia in inverted urothelial papillomas: pathology and prognostic significance. Hum Pathol 2004;35:1499-504. [PubMed]
- Epstein JI, Amin MB, Reuter VE. Bladder Biopsy Interpretation. Philadelphia: Lippincott Williams & Wilkins; 2004:67-74.
- Witjes JA, van Balken MR, van de Kaa CA. The prognostic value of a primary inverted papilloma of the urinary tract. J Urol 1997;158:1500-5. [PubMed]
- Xiao L, Wang CF, Zhu XZ, et al. Urothelial hyperplastic lesion with endophytic growth pattern: a clinicopathologic study. Zhonghua Bing Li Xue Za Zhi 2011;40:319-23. [PubMed]
- Zhang YT, Huang J, Li F, et al. Clinicopathologic analysis of urothelial carcinoma with an endophytic growth pattern. Pra J Clin Med 2012;9:109-111.
- Ho H, Chen YD, Tan PH, et al. Inverted papilloma of urinary bladder: is long-term cystoscopic surveillance needed? A single center's experience. Urology 2006;68:333-6. [PubMed]
- Kilciler M, Bedir S, Erdemir F, et al. Evaluation of urinary inverted papillomas: a report of 13 cases and literature review. Kaohsiung J Med Sci 2008;24:25-30. [PubMed]
- Asano K, Miki J, Maeda S, et al. Clinical studies on inverted papilloma of the urinary tract: report of 48 cases and review of the literature. J Urol 2003;170:1209-12. [PubMed]
- Tian BL, Gao YF, Xu S. The clinicopathologic analysis of non-invasive inverted urothelial neoplasms. J ClinExpPathol 2010;26:242-244.
- Amin MB, McKenney JK, Paner GP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Pathology. Eur Urol 2013;63:16-35. [PubMed]
- Epstein JI, Amin MB, Reuter VE. Urothelial neoplasms with inverted growth patterns. In: Biopsy Interpretation of the Bladder. Philadelphia: Lippincott Williams & Wilkins; 2010:79-93
- McKenney JK, Amin MB. Papillary Urothelial Neoplasm of Low Malignant Potential. In: Diagnostic Pathology: Genitourinary, Amirsys; 2010:76-9.
- Montironi R, Cheng L, Lopez-Beltran A, et al. Inverted (endophytic) noninvasive lesions and neoplasms of the urothelium: the Cinderella group has yet to be fully exploited. Eur Urol 2011;59:225-30. [PubMed]
- Maxwell JP, Wang C, Wiebe N, et al. Long-term outcome of primary Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP) including PUNLMP with inverted growth. Diagn Pathol 2015;10:3. [PubMed]
- Urakami S, Igawa M, Shirakawa H, et al. Inverted papilloma of the urinary bladder: a case evaluated for malignant potential. Int Urol Nephrol 1997;29:181-7. [PubMed]
- Sudo T, Irie A, Ishii D, Satoh E, et al. Histopathologic and biologic characteristics of a transitional cell carcinoma with inverted papilloma-like endophytic growth pattern. Urology 2003;61:837. [PubMed]
- Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol 2003;169:2101-5. [PubMed]
- Lazarevic B, Garret R. Inverted papilloma and papillary transitional cell carcinoma of urinary bladder: report of four cases of inverted papilloma, one showing papillary malignant transformation and review of the literature. Cancer 1978;42:1904-11. [PubMed]
- Terai A, Tamaki M, Hayashida H, et al. Bulky transitional cell carcinoma of bladder with inverted proliferation. Int J Urol 1996;3:316-9. [PubMed]
- Stern JB. Unusual benign bladder tumor of Brunn nest origin. Urology 1979;14:288-9. [PubMed]