The pathology of unusual subtypes of prostate cancer
The incidence of prostate cancer (PCa) is the first in malignant tumors among men in the United States, and the mortality rate is the second cause of cancer related deaths. The overwhelming majority of PCas are acinar adenocarcinomas (AC), which are mostly frequently encountered in our daily diagnostic work. Moreover, there are some unusual histological variants of prostate, because of their rarity, it is important for pathologists to recognize and make diagnosis accurately. In the 1973–2008 Surveillance, Epidemiology and End Results (SEER) program data, there were 790,937 acinar adenocarcinoma, and 806 cases of mucinous adenocarcinoma, 662 cases of ductal adenocarcinoma, 130 cases of signet ring cell carcinoma, 502 cases of neuroendocrine (NE) carcinoma, and 27 cases of adenosquamous carcinoma in US (1). Here, we mainly described the unusual histological variants of prostate adenocarcinoma, including atrophic, pseudo hyperplastic carcinomas, foamy gland, mucinous (colloid) carcinoma, signet ring cell carcinoma, basal cell and adenoid cystic carcinoma (ACC), squamous cell carcinoma (SQCC), ductal carcinoma, PCa with NE differentiation, and sarcomatoid carcinoma (Table 1).
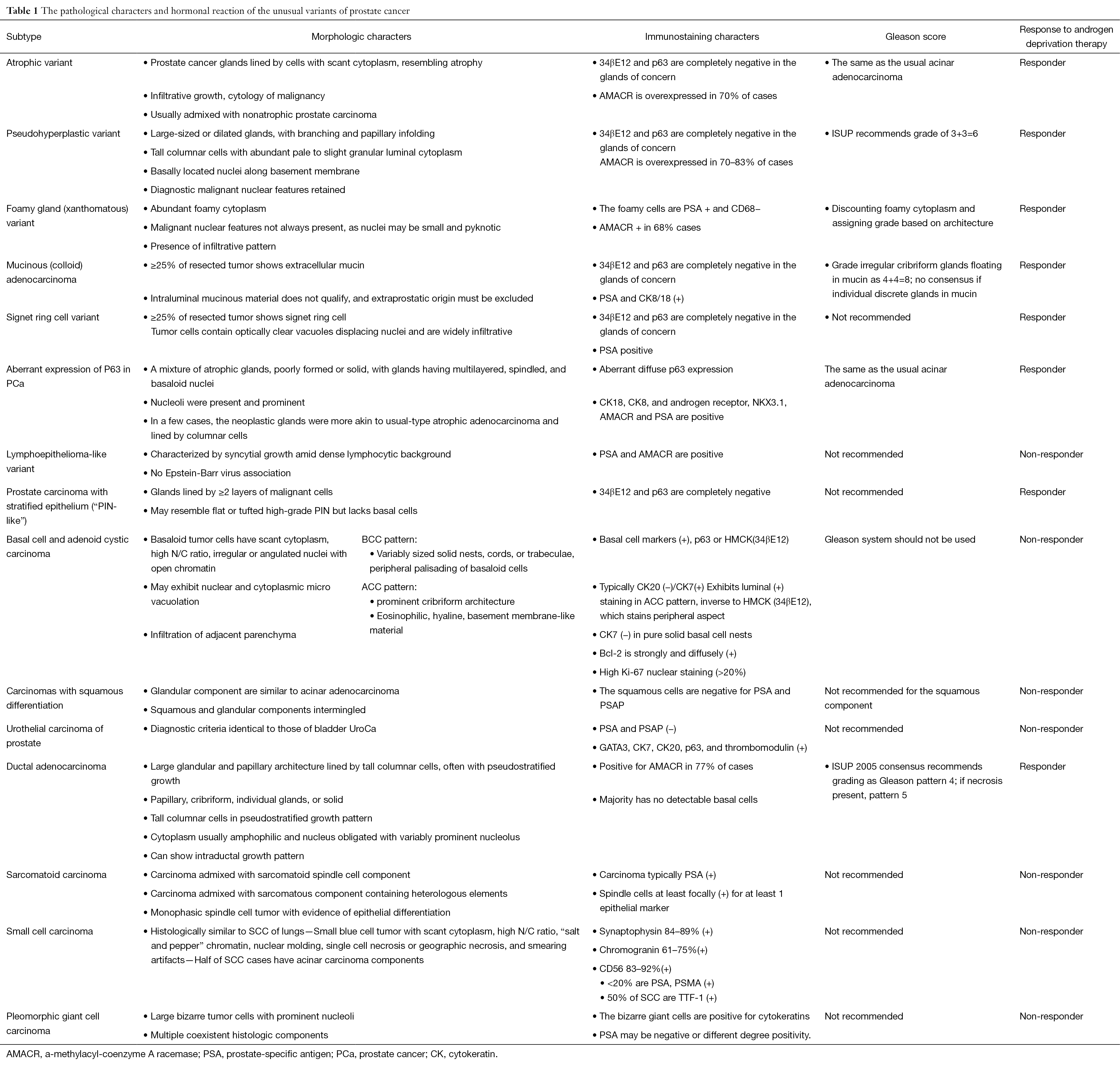
Full table
Acinar adenocarcinoma morphologic variants
These variants may have the same response to the hormone deprivation therapy as usual acinar adenocarcinoma. But, there may be distinctive morphologic change different from acinar adenocarcinoma, imitating the atrophy glands, foamy cells or usual hyperplastic glands. Sometimes, it is difficult to be differentiated from benign lesions, and leading to the missed diagnosis. Furthermore, some variants have different Gleason grading score, and different prognosis.
Atrophic variant
Atrophy in adenocarcinoma is identified with glands lined by cells with scant cytoplasm, which can simulate the appearance of atrophy in the prostate. Useful features in diagnostic recognition include infiltrative growth pattern and cytology of malignancy, the presence of macro nucleoli, nucleomegaly, and the presence of adjacent, non-atrophic prostatic carcinoma with the usual moderate amount of cytoplasm (2). In contrast, benign atrophic glands typically have dense hyperchromatic nuclei and lobular growth. High molecular weight cytokeratins (CKs) (34βE12) and p63 are completely negative in the glands of concern, and a-methylacyl-coenzyme A racemase (AMACR) is overexpressed in 70% of cases (3). It should be emphasis that focal basal cell loss and AMACR overexpression should be interpreted with caution, as this pattern can be seen in benign atrophic glands (4). Atrophic variant does not affect the Gleason grade or pathological stage (5), so it does not appear to have a different prognosis from usual acinar adenocarcinoma.
Pseudohyperplastic variant
Pseudohyperplastic adenocarcinoma is a variant characterized by large-sized or dilated glands, with branching and papillary infolding, tall columnar cells with abundant pale to slight granular luminal cytoplasm, which resemble the appearance of epithelial hyperplasia in the prostate (2,6,7). However, the abnormal architecture can be seen in majority of pseudohyperplastic carcinoma cases (2,8,9), such as infiltrative growth configuration, intraluminal crystalloids, perineural invasion, and intraluminal wispy blue mucin. In contrast to benign hyperplastic glands, diagnostic malignant nuclear features retained, including macro nucleoli, and complete absence of basal cells are present in every case.
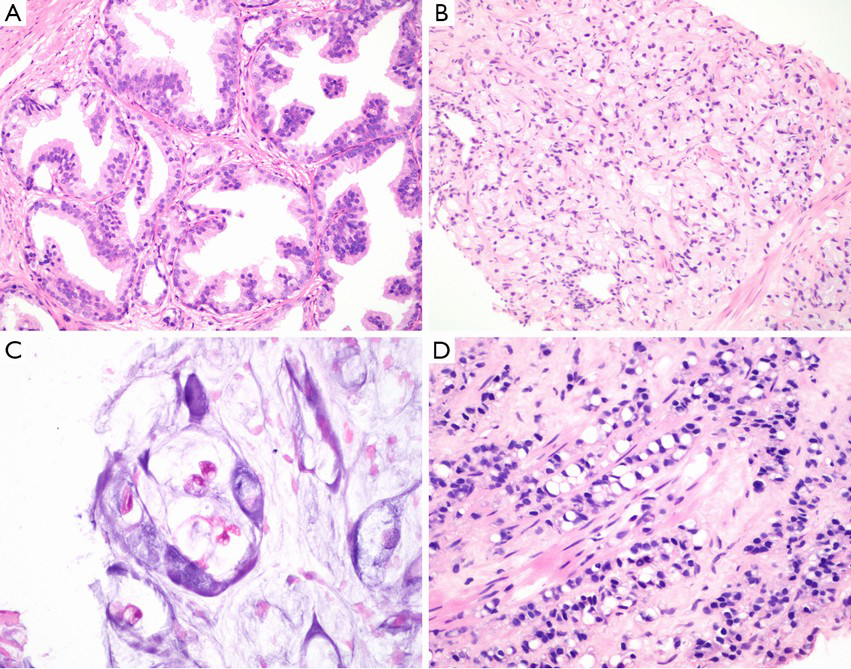
It should be with great caution when the diagnosis of pseudohyperplastic prostatic adenocarcinoma made in needle core cases. It is recommended to perform immunohistochemical stains for basal cells (34βE12 and⁄or p63) and AMACR in all suspect cases. It was reported that 70–83% of pseudohyperplastic prostatic adenocarcinoma cases are positive for AMACR by immunostaining (7) (Figure 1A).
ISUP recommends grade of 3+3=6 for pseudohyperplastic carcinoma. It was also recommended grade group of 1 accepted by the World Health Organization (10). The prognosis for patients with pseudohyperplastic prostatic adenocarcinoma is not certain, so clinical follow-up studies will be necessary to define the true prognostic impact of a pseudohyperplastic component.
Foamy gland (xanthomatous) variant
Foamy gland variant is characterized by abundant foamy cytoplasm. Malignant nuclear features not always present, as nuclei may be small and pyknotic (11,12). There is an architectural infiltrative pattern. Peripheral nerve invasion was more common in foamy gland carcinoma compared to acinar type adenocarcinoma (13). Foamy gland carcinoma may be deceptively benign appearing and missed on needle biopsy. Immunohistochemical stains are required in some cases for diagnosis, with prostate-specific antigen (PSA) and CD68 antibodies demonstrate the foamy cell are derived from prostate gland, and with 34βE12 and ⁄ or p63 antibodies demonstrate the absence of a basal cell layer in all cases of invasive foamy gland carcinoma. AMACR expression can be detected by immunohistochemistry in 68% foamy gland carcinomas (7),these stainings are useful to differential from xanthoma (4,13). ERG immunohistochemistry could be considered in a second round of immunostaining of select difficult cases of foamy gland carcinoma with low AMACR expression (14) (Figure 1B). ISUP recommends discounting foamy cytoplasm and assigning grade based on architecture, most are 3+3=6 tumors.
Clinically, patients with foamy gland carcinoma are of age ranging 50–78 years (15). Foamy gland carcinomas are of similar age, pathological stage, Gleason score as conventional acinar type adenocarcinoma, and may exhibit extraprostatic extension with foamy gland features (13,16).
Mucinous (colloid) adenocarcinoma
Mucinous adenocarcinoma of the prostate is defined as adenocarcinoma with at least 25% of resected tumor shows extracellular mucin, with exclusion of a non-prostatic origin (Figure 1C). Intraluminal mucinous material does not qualify. This carcinoma is one of the rare prostatic carcinoma variants, with an incidence of 0.3% of all prostatic carcinomas (17-19). If mucinous lakes are detected in needle biopsy, the recommended diagnosis is “adenocarcinoma with mucinous features”. If <25% of the tumor in radical prostatectomy sections is mucinous, one can make diagnosis of adenocarcinoma with focal mucin. With strict diagnostic criteria, it is suggested to have similar features and behavior with conventional PCa (17,18). PSA and CK8/18 antibodies demonstrate the mucinous cell are derived from prostate gland, other than metastasizing.
ISUP recommend to grade irregular cribriform glands floating in mucin as 4+4=8; no consensus if individual discrete glands in mucin. Two recent reports indicated that mucinous adenocarcinoma treated by radical prostatectomy might even be less aggressive than usual acinar adenocarcinoma (17,18).
Signet ring cell variant
Signet ring carcinoma is not an exactly definited rare prostate adenocarcinoma type, with the required percentage of the tumor to be signet ring cells ranging from 25% to 50% in the literature True signet ring cell carcinomas should have intracytoplasmic mucin, optically clear vacuoles displacing nuclei and are widely infiltrative (20-23) (Figure 1D). Moreover, it should be identified with vacuolated adenocarcinoma of the prostate. Most cases are signet ring cell-like adenocarcinoma (20). There may be mucin-producing PCa, named “mucinous carcinoma with signet ring cells”. It is not clear if nonmucinous (mucicarmine negative) signet ring PCa is distinct clinically.
The true incidence of prostate signet ring carcinoma is not known. The prognosis for patients with signet ring carcinoma is poorer than conventional prostate carcinoma, and less response to the hormonal treatment.
Aberrant expression of P63 in PCa
Prostatic carcinoma with aberrant diffuse p63 expression is a recently described rare unusual variant of PCa. It is important to avoid making misdiagnosis as benign acinar proliferation. The differential diagnosis includes basal cell hyperplasia and basal cell carcinoma (24). The mean age of the patients was 65 years (range, 50–81 years old) (25). The morphologic change is consist of a mixture of atrophic glands, some poorly formed or solid, with glands having multilayered, spindled, and basaloid nuclei. Nucleoli were present and prominent in the majority of the neoplastic glands (26). In a few cases, the neoplastic glands had no distinctive morphology and were more akin to usual-type atrophic adenocarcinoma without nuclear palisading or basaloid morphology or showed neoplastic glands lined by columnar cells. In 19/21 cases, 100% of the cancer nuclei stained intensely for p63, with 70% staining in the remaining 2 cases (25). Despite p63 positivity, these tumors uniformly expressed luminal-type cytokeratin proteins such as CK18 (13/13), CK8 (8/8), and markers of androgen axis signaling commonly seen in luminal cells, including androgen receptor (10/11), NKX3.1 (8/8), and protein (12/13) (27). In 13/13 cases studied, tumor was positive for AMACR and in 12/12 cases (100%) were positive for PSA. Mean ki-67 expression was 6.25% (range, 5% to 10%) in 7 cases (25). In contrast to usual prostatic AC, p63-PCa shows a mixed luminal/basal immunophenotype, uniformly lack ERG gene rearrangement, and frequently express GSTP1 (27). Gleason grading of this rare variant of PCa is controversial at present. About 80% cases are graded as Gleason 3+X, while about 20% cases as Gleason 4+X and 5+X (26).
PCa with stratified epithelium (“PIN-like”)
Prostate intraepithelialneoplasia (PIN)-like adenocarcinoma is described as glands lined by more than 2 layers of malignant cells that resembling PIN in gland architecture (28,29). It may resemble flat or tufted high-grade PIN but lacks basal cells by immunohistochemical stain for 34βE12 and p63. AMACR staining can be a positive. Some may have high columnar cells like ductal AC. The reported clinical follow-up is at only 5 months (29) and so its prognosis is not definite.
Other rarer variants
These rare variants have quite different clinical and pathological characteristics with acinar adenocarcinoma, they may represent the “true” subtypes, since many of them do not respond to hormone deprivation treatment, it is very important to recognize these variants.
Lymphoepithelioma-like variant
Lymphoepithelioma-like variant are very rare, poorly differentiated. Histologically, they resemble lymphoepithelioma in other organs, characterized by syncytial growth amid dense lymphocytic background, but do not appear to be related to Epstein-Barr virus infection. The carcinoma cells are positive for PSA and AMACR. The prognosis is poor. It is reported that patients died of the cancer at 8–26 months after diagnosis (30).
Basal cell and ACC
Basal cell lesions in the prostate gland span a wide range from obviously benign basal cell hyperplasia through varying ranges of atypia to lesions. A precise definition of malignant features include elusive, haphazard infiltrative growth, perineural invasion, extraprostatic extension—with the bladder neck as a preferred site, the presence of necrosis are widely accepted features (31-33). Clinically, it mainly occurred in older men, most commonly presents with obstructive urinary symptoms; it has reported that PSA is elevated in some cases (34). Local recurrence, metastasis, and death from disease reported in approximately 30% of cases. Given limited case numbers with clinical outcome data, the varying ranges of atypical lesions can be viewed as a low malignant potential neoplasm.
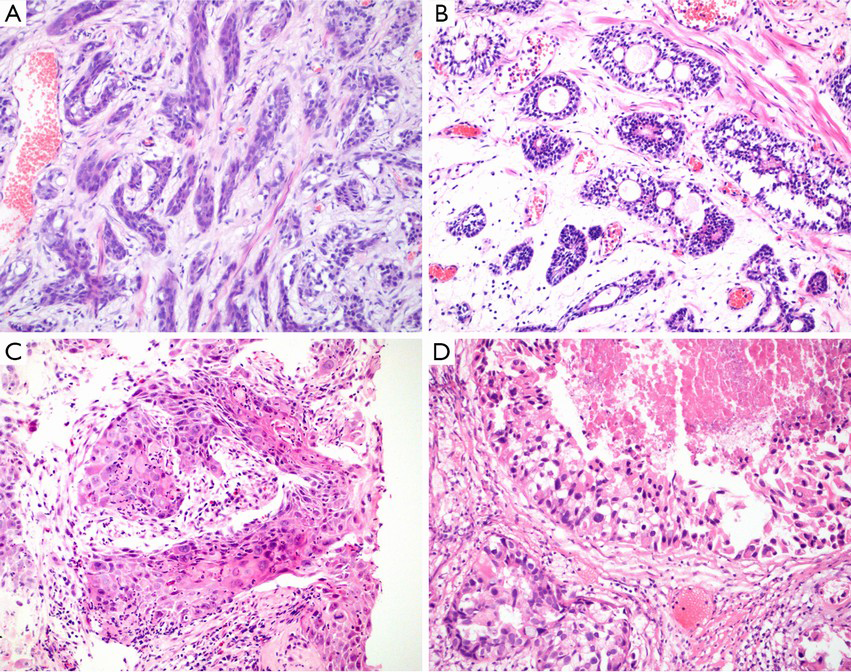
Basaloid tumor cells have scant cytoplasm, high nuclear to cytoplasmic ratio, and irregular or angulated nuclei with open chromatin. Most experts recognize malignant “basaloid” neoplasms as either basal cell or ACC (31,33). Basal cell carcinoma (BCC) pattern, composed of variably sized solid nests, cords, or trabeculae with peripheral palisading of basaloid cells, may be associated with extensive central necrosis. Basal cell carcinoma of the prostate is an aggressive tumor with frequent loss of PTEN expression and overexpression of EGFR (35) (Figure 2A). ACC pattern, in which tumor grows in nests with prominent cribriform architecture, and eosinophilic, hyaline, basement membrane-like material may be present. Basophilic mucinous secretions may be presented in lumina (Figure 2B). Histologically, basaloid carcinomas of the prostate were considered to originate from basal/reserve cells, not the same as the usual PCa (31,33). Recently, it was considered that true ACCs often harbor MYB translocations resulting in the MYB-NFIB fusion protein (36). The Gleason system should not be applied to these tumors.
The main differential diagnoses are primary benign or atypical basal cell hyperplasia. In resolving challenging cases, it is important to recognize the nuclear atypia (33). Some adenoid cystic-like proliferations may be multifocal, exhibit focal cribriform architecture, but they typically show well-defined lobulated growth patterns, lacking the confluent, expansile cribriform architecture or widely irregular infiltrative patterns of basaloid carcinomas (37). In addition to assessment of architectural features such as infiltrative growth, perineural invasion, extraprostatic extension and cytologic features, IHC for p63, HMCK (34βE12), CK7, CK20, Bcl-2 and Ki67 may be helpful to distinguish basal cell hyperplasia (BCH) from basaloid carcinoma. Basal cell markers, p63 or HMCK (34βE12) may stain multiple layers of cells, only peripheral aspect of tumor clusters, or in only few scattered tumor cells. CK7 exhibits luminal positive staining in ACC pattern, inverse to HMCK (34βE12), which stains peripheral aspect, while CK7 is negative in pure solid basal cell nests. Unlike usual PCa and BCH, in which Bcl-2 staining is absent or is focal/weak, basaloid carcinomas often demonstrate diffuse Bcl-2 positivity. The proliferative rate for basaloid carcinomas is always >20%, significantly higher than that of BCH (38).
Carcinomas with squamous differentiation
The prostatic pure SQCC is similar to SQCC of other anatomic sites, which showed well to poorly differentiating with variable cytologic atypia (Figure 2C). Glandular component of prostatic adenosquamous cell carcinoma (ASC) is similar to acinar adenocarcinoma. Squamous and glandular components intermingled. If there is no history of hormonal therapy, the glandular component of adenosquamous carcinomas can be Gleason graded. But the squamous component can show variable differentiation and not be graded using the Gleason system. The malignant squamous cells are most often negative for PSA and PSAP immunostains. The mean survival for prostatic SQCC is not long, about 6–24 months (39).
Urothelial carcinoma of prostate
Urothelial Carcinoma of Prostate (UroCa) involving prostate arise from prostatic urethra, periurethral glands, and proximal prostatic ducts. Prostate involvement by invasive bladder UroCa is relatively more common. Most primary prostatic UroCa presents with obstructive urinary symptoms. Diagnostic criteria are identical to those of bladder UroCa. Cytologically, urothelial carcinoma in the prostate has a high nuclear grade, with substantial nucleomegaly, nuclear pleomorphism, and nuclear hyperchromasia. The cytoplasm often has an eosinophilic ‘squamoid’ appearance (Figure 2D). Carcinoma in situ of UroCa can spread from prostatic urethra, involves ducts and acini, or grows along ejaculatory duct to seminal vesicle, stromal invasion may arise anywhere along this spread. Prostatic stromal invasion by urothelial carcinoma is typical fied by irregular solid nests and cords that extend beyond the rounded, smooth outer profile of the urothelial carcinoma in situ, with a fibroinflammatory stromal response (30).
The immunohistochemistry stain is useful to differentiate the urothelial carcinoma from poorly differentiated prostatic adenocarcinoma. PSA and PSAP staining is negative for the urothelial carcinoma, CK7, CK20, high molecular weight CKs bound by 34βE12 (CK903), p63, and thrombomodulin are frequently positive (30). GATA3 is positive in the UroCa and negative in the prostatic adenocarcinoma, it is a good marker to identify the urothelial origin (4,40).
Clinically, Serum PSA level of UroCa typically is not elevated. Grading and pathological staging have been inconsistently performed. The outcome for patients diagnosed with primary urothelial carcinoma of the prostate has been dismal in the past, with an average survival of 17–29 months (41).
Ductal adenocarcinoma
Prostatic ductal adenocarcinoma (PDA) is characterized as adenocarcinoma of prostatic epithelial cell origin with large glandular and papillary architecture lined by tall columnar cells, often with pseudostratified growth. Histologically, main architectural patterns include papillary, cribriform, individual glands and solid. Cytoplasm usually amphophilic and nucleus obligated with variably prominent nucleolus. It can grow intraluminally into preexisting ducts retaining “native” basal cell layer. Cytologically, in ductal adenocarcinoma, nucleus typically elongated, variably usually prominent nucleolus, occasional to frequent mitoses present. It has greater degree of chromatin irregularities compared to usual acinar prostate adenocarcinoma (Figure 3A,B).

Clinically, mixed ductal and acinar adenocarcinoma was reported in 5–6.3% of PCas (42). It is considered that PDA and the synchronous usual-type acinar adenocarcinoma are clonally related in some cases and are infrequent PTEN loss and ERG protein expression (43). Patients with PDA presented with lower PSA, had more favorable pathological features, and similar overall survival compared to men with Gleason 8–10 acinar adenocarcinoma (44). It is reported that PDA respond to hormonal manipulation treatment.
PDA has morphological similarities to AC of other organs. It is important for therapeutic decisions to distinguish the prostatic origin with the metastases from other organs. Androgen receptor, protein, PSA, and PSAP were almost invariably expressed in PDA. Ki-67-labeling index was lower in PDA than in other AC (4,45,46). Positive rate of AMACR is in 77% of cases. Majority has no detectable basal cells by p63 or HMCK (34βE12). ISUP 2005 consensus recommends grading as Gleason pattern 4; if necrosis present, pattern 5.
Sarcomatoid carcinoma
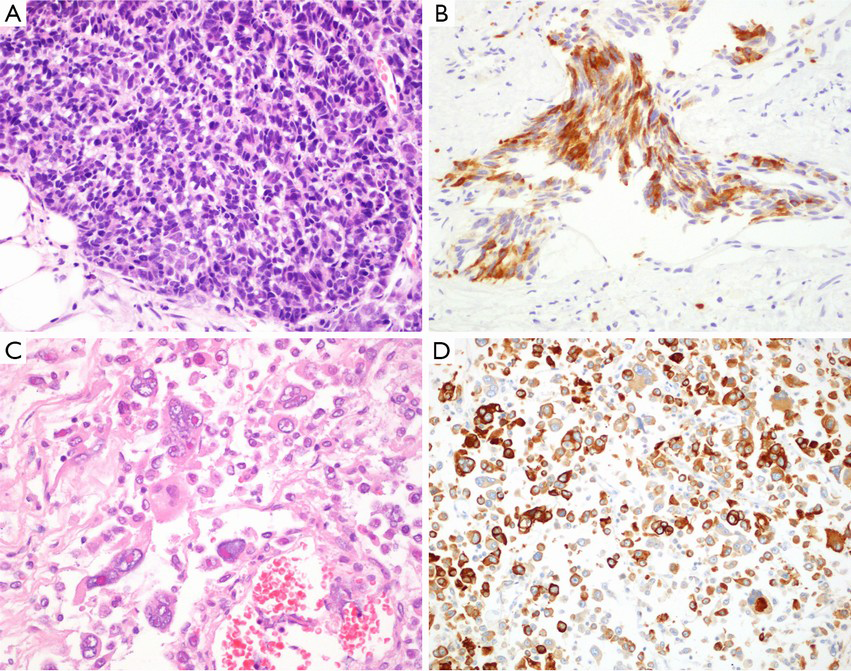
Sarcomatoid carcinoma is a rare biphasic malignant or monophasic neoplasm of prostate demonstrating epithelial and mesenchymal differentiation by light microscopic or immunohistochemistry. Two elements of sarcomatoid carcinoma include a malignant epithelial component and a malignant mesenchymal or mesenchymal-like component. Overall, histologically, sarcomatoid carcinoma falls into three categories. The most common one is carcinoma admixed with sarcomatoid spindle cell component. The other is carcinoma admixed with sarcomatous component containing heterologous elements. The third one is the monophasic spindle cell tumor with immunohistochemical and/or electron microscopic evidence of epithelial differentiation (Figure 3C,D). Sarcomatoid component frequently composed of undifferentiated spindle cell sarcoma. About 24% cases may contain heterologous elements that most frequently resemble osteosarcoma, or leiomyosarcoma, chondrosarcoma, rhabdomyosarcoma, and angiosarcoma. The combination of these sarcomatous elements is common. Heterologous elements typically merge with spindle cells. IHC findings in the 2 components are different, with adenocarcinoma showing diffuse CK and PSA/PAP positivity and the spindle cells exhibiting more focal CK positivity and rare PSA/PAP labeling (47-49). The prognosis of prostatic sarcomatoid carcinoma is poor with local recurrence not response to hormonal treatment (50).
Small cell carcinoma (SmCC)
Primary SmCC of prostate is very rare, the estimated incidence range from 0.3% to 1% of all prostatic carcinomas. In about one-half of cases, the carcinoma is pure SmCC, and in the other half it is admixed with prostatic acinar adenocarcinoma (33). SmCC is histologically similar to SmCC of lungs, including small blue cell tumor with scant cytoplasm, high nuclear to cytoplasmic ratio, “salt and pepper” chromatin, nuclear molding, single cell necrosis or geographic necrosis, and smearing artifacts (Figure 4A,B). Focal NE differentiation in acinar adenocarcinoma is characterized by scattered or focal cluster of NE cells mixing with adenocarcinoma cellular elements, highlighted by NE markers. Paneth cell-like change may occur (51).
Hormonal treatment or androgen deprivation therapy (ADT) for acinar adenocarcinoma may lead NE tumor cells to clonal propagation, for NE cells are devoid of androgen receptors; Possible clonal progression and evolution of subset of non-NE tumor cells that have been influenced by ADT (51).
It is reported that NE marker immunoreactivity in SmCC, that synaptophysin positive in 84–89% cases, chromogranin positive 61–75% cases, CD56 positive in 83–92% cases, NSE positive in 85% cases, and prostate specific markers (PSA, PAP, PSMA) positive in less than 20% cases. Approximately 50% of SmCCs are TTF-1 positive. So, it will be difficult to identify the derivation of the SmCC found in prostate. The test of TMPRSS2-ERG Gene Fusion in SmCC of Prostate will be helpful. TMPRSS2 (transmembrane proteinase serine 2) is an androgen-regulated gene that is specifically expressed in the prostate. There is androgen responsive element in the 5’ untranslated region of the TMPRSS2 gene. Therefore, the fusion of 5’ TMPRSS2 to 3’ERG leads to the expression of ERG under the androgen control. Overall, in the prostate, the TMPRSS2-ERG gene fusion is seen in 50% (40–70%) of prostate adenocarcinoma, and is also reported in about 20% of high-grade PIN (52). But it is generally negative in the benign glands. On FISH analysis, the rearrangement of the ERG gene was found in 8 cases of prostatic SmCC (67%), and the rearrangement was associated with deletion of the 5’ ERG gene in 7 cases, but rearrangement of the ERG gene was not present in any SmCC of the urinary bladder (12 cases) or lung (11 cases) (52). So the FISH analysis of TMPRSS2-ERG gene rearrangement is helpful in identify the derivation of the SmCC in prostate.
Pleomorphic giant cell CA
This is a very rare variant of adenocarcinoma with large bizarre tumor cells with prominent nucleoli. In addition to the pleomorphic giant cell component, multiple coexistent histologic components may be seen including Gleason score 9 conventional PCa, SmCC, squamous carcinoma, and prominent ductal adenocarcinoma differentiation with intraductal spread (53). In immunohistochemical staining, PSA may be negative in the giant cells, or different degree positivity. The bizarre giant cells were strongly positive for cytokeratins AE1/AE3 and/or Cam 5.2 (Figure 4C,D). It is often more aggressive conventional PCa in clinical outcome (53).
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China No.81570180, 81072103 (to Dr. Wang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marcus DM, Goodman M, Jani AB, et al. A comprehensive review of incidence and survival in patients with rare histological variants of prostate cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis 2012;15:283-8. [PubMed]
- Arista-Nasr J, Martínez-Benítez B, Aguilar-Ayala EL, et al. Pseudohyperplastic prostate carcinoma: histologic patterns and differential diagnosis. Ann Diagn Pathol 2015;19:253-60. [PubMed]
- Farinola MA, Epstein JI. Utility of immunohistochemistry for alpha-methylacyl-CoA racemase in distinguishing atrophic prostate cancer from benign atrophy. Hum Pathol 2004;35:1272-8. [PubMed]
- Epstein JI, Egevad L, Humphrey PA, et al. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol 2014;38:e6-e19. [PubMed]
- Kaleem Z, Swanson PE, Vollmer RT, et al. Prostatic adenocarcinoma with atrophic features: a study of 202 consecutive completely embedded radical prostatectomy specimens. Am J Clin Pathol 1998;109:695-703. [PubMed]
- Levi AW, Epstein JI. Pseudohyperplastic prostatic adenocarcinoma on needle biopsy and simple prostatectomy. Am J Surg Pathol 2000;24:1039-46. [PubMed]
- Zhou M, Jiang Z, Epstein JI. Expression and diagnostic utility of alpha-methylacyl-CoA-racemase (P504S) in foamy gland and pseudohyperplastic prostate cancer. Am J Surg Pathol 2003;27:772-8. [PubMed]
- So JS, Epstein JI. Histologic features of pseudohyperplastic perineural invasion in prostatic adenocarcinoma: a mimicker of benign hyperplastic glands and high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol 2014;38:852-7. [PubMed]
- Zhang HZ, Jiang ZM, Shi L. Pathologic characteristics of pseudohyperplastic prostatic adenocarcinoma. Zhonghua Bing Li Xue Za Zhi 2007;36:742-5. [PubMed]
- Epstein JI. Prostate Cancer Grading: A Contemporary Photomontage. Am J Surg Pathol 2016;40:137. [PubMed]
- Tran TT, Sengupta E, Yang XJ. Prostatic foamy gland carcinoma with aggressive behavior: clinicopathologic, immunohistochemical, and ultrastructural analysis. Am J Surg Pathol 2001;25:618-23. [PubMed]
- Zhao J, Epstein JI. High-grade foamy gland prostatic adenocarcinoma on biopsy or transurethral resection: a morphologic study of 55 cases. Am J Surg Pathol 2009;33:583-90. [PubMed]
- Koca SB, Yıldız P, Behzatoğlu K. Foamy gland carcinoma in core needle biopsies of the prostate: clinicopathologic and immunohistochemical study of 56 cases. Ann Diagn Pathol 2014;18:271-4. [PubMed]
- Warrick JI, Humphrey PA. Foamy gland carcinoma of the prostate in needle biopsy: incidence, Gleason grade, and comparative α-methylacyl-CoA racemase vs. ERG expression. Am J Surg Pathol 2013;37:1709-14. [PubMed]
- Nelson RS, Epstein JI. Prostatic carcinoma with abundant xanthomatous cytoplasm. Foamy gland carcinoma. Am J Surg Pathol 1996;20:419-26. [PubMed]
- Hudson J, Cao D, Vollmer R, et al. Foamy gland adenocarcinoma of the prostate: incidence, Gleason grade, and early clinical outcome. Hum Pathol 2012;43:974-9. [PubMed]
- Lane BR, Magi-Galluzzi C, Reuther AM, et al. Mucinous adenocarcinoma of the prostate does not confer poor prognosis. Urology 2006;68:825-30. [PubMed]
- López JI, Laforga JB. Mucinous (colloid) adenocarcinoma of the prostate. Br J Urol 1995;76:805-6. [PubMed]
- Dhom G. Unusual prostatic carcinomas. Pathol Res Pract 1990;186:28-36. [PubMed]
- Hejka AG, England DM. Signet ring cell carcinoma of prostate. Immunohistochemical and ultrastructural study of a case. Urology 1989;34:155-8. [PubMed]
- Alline KM, Cohen MB. Signet-ring cell carcinoma of the prostate. Arch Pathol Lab Med 1992;116:99-102. [PubMed]
- England DM, Hejka AG. Signet-ring cell carcinoma of the prostate--always an aggressive lesion? Arch Pathol Lab Med 1992;116:812. [PubMed]
- Fujita K, Sugao H, Gotoh T, et al. Primary signet ring cell carcinoma of the prostate: report and review of 42 cases. Int J Urol 2004;11:178-81. [PubMed]
- Wu A, Kunju LP. Prostate cancer with aberrant diffuse p63 expression: report of a case and review of the literature and morphologic mimics. Arch Pathol Lab Med 2013;137:1179-84. [PubMed]
- Osunkoya AO, Hansel DE, Sun X, et al. Aberrant diffuse expression of p63 in adenocarcinoma of the prostate on needle biopsy and radical prostatectomy: report of 21 cases. Am J Surg Pathol 2008;32:461-7. [PubMed]
- Giannico GA, Ross HM, Lotan T, et al. Aberrant expression of p63 in adenocarcinoma of the prostate: a radical prostatectomy study. Am J Surg Pathol 2013;37:1401-6. [PubMed]
- Tan HL, Haffner MC, Esopi DM, et al. Prostate adenocarcinomas aberrantly expressing p63 are molecularly distinct from usual-type prostatic adenocarcinomas. Mod Pathol 2015;28:446-56. [PubMed]
- Hameed O, Humphrey PA. Stratified epithelium in prostatic adenocarcinoma: a mimic of high-grade prostatic intraepithelial neoplasia. Mod Pathol 2006;19:899-906. [PubMed]
- Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasialike ductal adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol 2008;32:1060-7. [PubMed]
- Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology 2012;60:59-74. [PubMed]
- Epstein JI, Armas OA. Atypical basal cell hyperplasia of the prostate. Am J Surg Pathol 1992;16:1205-14. [PubMed]
- Iczkowski KA, Ferguson KL, Grier DD, et al. Adenoid cystic/basal cell carcinoma of the prostate: clinicopathologic findingBasal cell carcinoma of the prostate: a clinicopathologic study of 29 cases.zs in 19 cases. Am J Surg Pathol 2003;27:1523-9. [PubMed]
- Ali TZ, Epstein JI. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. Am J Surg Pathol 2007;31:697-705. [PubMed]
- Terris MK. The appearance of adenoid cystic carcinoma of the prostate on transrectal ultrasonography. BJU Int 1999;83:875-6. [PubMed]
- Simper NB, Jones CL, MacLennan GT, et al. Basal cell carcinoma of the prostate is an aggressive tumor with frequent loss of PTEN expression and overexpression of EGFR. Hum Pathol 2015;46:805-12. [PubMed]
- Bishop JA, Yonescu R, Epstein JI, et al. A subset of prostatic basal cell carcinomas harbor the MYB rearrangement of adenoid cystic carcinoma. Hum Pathol 2015;46:1204-8. [PubMed]
- McKenney JK, Amin MB, Srigley JR, et al. Basal cell proliferations of the prostate other than usual basal cell hyperplasia: a clinicopathologic study of 23 cases, including four carcinomas, with a proposed classification. Am J Surg Pathol 2004;28:1289-98. [PubMed]
- Yang XJ, McEntee M, Epstein JI. Distinction of basaloid carcinoma of the prostate from benign basal cell lesions by using immunohistochemistry for bcl-2 and Ki-67. Hum Pathol 1998;29:1447-50. [PubMed]
- Nabi G, Ansari MS, Singh I, et al. Primary squamous cell carcinoma of the prostate: a rare clinicopathological entity. Report of 2 cases and review of literature. Urol Int 2001;66:216-9. [PubMed]
- Hoang LL, Tacha D, Bremer RE, et al. Uroplakin II (UPII), GATA3, and p40 are Highly Sensitive Markers for the Differential Diagnosis of Invasive Urothelial Carcinoma. Appl Immunohistochem Mol Morphol 2015;23:711-6. [PubMed]
- Greene LF, O'Dea MJ, Dockerty MB. Primary transitional cell carcinoma of the prostate. J Urol 1976;116:761-3. [PubMed]
- Colpaert C, Gentens P, Van Marck E. Ductal ("endometrioid") adenocarcinoma of the prostate. Acta Urol Belg 1998;66:29-32. [PubMed]
- Morais CL, Herawi M, Toubaji A, et al. PTEN loss and ERG protein expression are infrequent in prostatic ductal adenocarcinomas and concurrent acinar carcinomas. Prostate 2015;75:1610-9. [PubMed]
- Packiam VT, Patel SG, Pariser JJ, et al. Contemporary Population-Based Comparison of Localized Ductal Adenocarcinoma and High-Risk Acinar Adenocarcinoma of the Prostate. Urology 2015;86:777-82. [PubMed]
- Leite KR, Mitteldorf CA, Srougi M, et al. Cdx2, cytokeratin 20, thyroid transcription factor 1, and prostate-specific antigen expression in unusual subtypes of prostate cancer. Ann Diagn Pathol 2008;12:260-6. [PubMed]
- Seipel AH, Samaratunga H, Delahunt B, et al. Immunohistochemistry of ductal adenocarcinoma of the prostate and adenocarcinomas of non-prostatic origin: a comparative study. APMIS 2016. [Epub ahead of print]. [PubMed]
- Ordoñez NG, Ayala AG, von Eschenbach AC, et al. Immunoperoxidase localization of prostatic acid phosphatase in prostatic carcinoma with sarcomatoid changes. Urology 1982;19:210-4. [PubMed]
- Hansel DE, Epstein JI. Sarcomatoid carcinoma of the prostate: a study of 42 cases. Am J Surg Pathol 2006;30:1316-21. [PubMed]
- Dundore PA, Cheville JC, Nascimento AG, et al. Carcinosarcoma of the prostate. Report of 21 cases. Cancer 1995;76:1035-42. [PubMed]
- Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol 2004;17:316-27. [PubMed]
- Gladell PP, Rafael EJ. Small Cell Carcinoma and Other Neuroendocrine Tumors. In: Mahul Amin B, eds. Diagnostic Pathology Genitourinary. Salt Lake City: AMIRSYS publishing 2010:3-114.
- Guo CC, Dancer JY, Wang Y, et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum Pathol 2011;42:11-7. [PubMed]
- Parwani AV, Herawi M, Epstein JI. Pleomorphic giant cell adenocarcinoma of the prostate: report of 6 cases. Am J Surg Pathol 2006;30:1254-9. [PubMed]
