Efficacy and safety of trastuzumab combined with chemotherapy for first-line treatment and beyond progression of HER2-overexpressing advanced breast cancer
Huiping Li, Bin Shao, Yin Yan, Guohong Song, Xiaoran Liu, Jing Wang, Xu Liang
Abstract
Objective: To observe the efficacy and safety of trastuzumab combined with chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)-overexpressing advanced breast cancer.
Methods: A total of 90 patients with HER2-overexpressing advanced breast cancer were enrolled in this study. All patients were diagnosed with ductal invasive breast cancer by pathological analysis, and were aged between 31–73 years with a median of 51 years. HER2-positivity was defined as 3(+) staining in immunochemistry or amplification of fluorescence in situ hybridization (FISH, ratio ≥2.0). Trastuzumab was administered in combination with chemotherapy as first-line treatment and beyond progression as a secondline, third-line, and above treatment in 90, 34, 14, and 6 patients, respectively. The chemotherapy regimen was given according to normal clinical practice. The response rate was evaluated every two cycles, and the primary endpoints were progression-free survival (PFS) and overall survival (OS). Survival curves were estimated by using Kaplan-Meier graphs and were compared by using log-rank test statistics. Multivariate analysis was done using Cox’s proportional hazards regression model, and the level of significance was P<0.05.
Results: All 90 patients received at least one dose of trastuzumab, and efficacy could be evaluated in 85 patients. The median follow-up was 50 months. In total, 72 (80.00%) patients had visceral metastasis, and 43 (47.78%) patients had progressed after one or more extensive chemotherapy regimens for metastatic diseases. The median PFS for first-line trastuzumab was 10 months (range, 2–59 months), and the median OS after metastasis or initially local advanced disease was 22 months (range, 2–116 months).
Conclusions: Trastuzumab combined with chemotherapy was active and well-tolerated as a first-line treatment and even beyond progression in HER2-overexpressing advanced breast cancer as a second-line or third-line treatment. However, its efficacy is certainly less beyond this point.
Keywords: Trastuzumab; chemotherapy; advanced breast cancer; efficacy and safety
Submitted Aug 20, 2015. Accepted for publication May 04, 2016.
doi: 10.21147/j.issn.1000-9604.2016.03.07
Introduction
The human epidermal growth factor receptor 2 gene (HER2) encodes a 185-kD transmembrane glycoprotein receptor (p185HER2) and is amplified in 25%-30% of human breast (1). Patients with HER2 overexpression exhibit shorter disease-free survival (DFS) and overall survival (OS) (2). Trastuzumab (Herceptin; F. Hoffmann-La Roche Ltd, Basel, Switzerland) is a humanized murine monoclonal antibody that binds specifically to the extracellular domain of the HER2 protein. The survival benefit from trastuzumab has beenwell established in numerous clinical trials of patients with early and metastatic breast cancer who had overexpression of HER2. The use of trastuzumab either in monotherapy or combination with chemotherapy (3-6) and endocrine therapy all resulted in survival benefit (7). Trastuzumab is most frequently combined with chemotherapy agents including paclitaxel, docetaxel, vinorelbine, gemcitabine and carboplatin (4-6, 8), and also provides a survival advantage to women who have been previously treated with chemotherapy for metastatic disease (9). Furthermore, efficacy has been observed following continuation of trastuzumab after trastuzumab progression according to data from a phase Ⅲ randomized study [German Breast Group (GBG)-26] (9). In China, the first clinical trial using trastuzumab in metastatic breast cancer was a phase Ⅱ trial published in 2003 (10). However, while the trastuzumab in combination with chemotherapy has been a standard regimen for over ten years, and its usage was limited by its high cost. However, more widespread use resulted from a public welfare project for trastuzumab was managed by the China Cancer Foundation in August 2011. Thus, in the past 2 or 3 years there has been no large-scale study of trastuzumab in advanced breast cancer in China. In this observational study, the majority of data were collected over the past two years (72/90 patients; 80.0%). Since pertuzumab and T-DM1 have not been approved in China, trastuzumab and lapatinib remain the only available anti-HER2 targeted therapies for Chinese patients.
In this article, we evaluated the outcome of patients with advanced breast cancer who received trastuzumab treatment in routine clinical practice. This observational study focused on the efficacy and safety of trastuzumab combined with chemotherapy for first-line treatment and beyond progression upon trastuzumab treatment of HER2-overexpressing advanced breast cancer.
Patients and methods
Patient selection
Women with advanced (either metastatic or locally advanced) measurable disease and HER2-overexpressing breast cancer who had at least one dose of trastuzumab were eligible for this study. All patients were confirmed with ductal invasive breast cancer by pathology upon initial diagnosis. HER2-positivity was defined as 3(+) staining in immunohistochemistry (IHC) or amplification of fluorescence in situ hybridization (FISH, ratio ≥2.0) if the IHC staining score was 2(+), a life expectancy ≥3 months and performance status [on the Eastern Cooperative Oncology Group (ECOG) scale] ≤2. In addition, a cardiac evaluation with echocardiography had to show an ejection fraction value ≥50% at baseline. Patients were excluded if they had clinically significant cardiac disease, infection, bleeding disorder, abnormal pulmonary function, or other significant medical conditions. The study was approved by the Ethics Committee of Beijing Cancer Hospital (Beijing, China) and informed consent was obtained from all patients before starting treatment.
The study observed 90 patients with HER2-overexpressing advanced breast cancer from January 2006 to September 2014, and there were 72/90 (80.0%) cases from September 2012 to September 2014. Nine of 90 (10.0%) patients were initially diagnosed with advanced breast cancer, and the rest 81 (90.0%) experienced metastatic diseases after breast cancer surgery, wherein 23/81 (28.4%) patients underwent re-biopsy at a metastatic tumor site. Patients were aged between 31-73 years (median, 51 years) at diagnosis.
Treatment regimen
Patients who had received either first-line trastuzumab combined with chemotherapy or trastuzumab treatment beyond progression were enrolled. Trastuzumab was given 4 mg/kg (in 82 patients) or 8 mg/kg (in 8 patients) loading dose, followed by 2 mg/kg weekly or by 6 mg/kg per 3 weeks respectively. One cycle was defined as 21 days.
The chemotherapy regimen was administered according to normal clinical practice and advanced breast cancer guidelines (11, 12), and trastuzumab was continued after first-line trastuzumab progression unless the patients refuse treatment for cost, side effects or other factors, in which case chemotherapy agents were changed from first-line chemotherapy agents.
Patient evaluation
History, physical examination, ECOG status, blood cell count and serum chemistry were assessed at baseline and repeated before each cycle. A left ventricular ejection fraction (LVEF) measurement was performed at baseline by echocardiography and reassessed every three months. Tumor measurement was performed by computed tomography (CT) scan or magnetic resonance imaging (MRI), and was repeated every two cycles of treatment (by 2 months), upon which pathology was reviewed, especially for patients who were not initially treated in the Department of Breast Oncology, Peking University Cancer Hospital.
The primary end point was progression-free survival (PFS). The secondary end points included OS, objective response rate (ORR), and adverse events (AEs). The tumor response rate was evaluated using the Response Evaluation Criteria in Solid Tumors criteria version 1.1 (13). The best response across theobservation was recorded. PFS and OS were calculated as the time from the first trastuzumab administration to progression and death at the last valid observation point.
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE 3.0) (14).
Statistical analysis
The PFS and OS at specific time points were estimated using SPSS software (version 19.1; SPSS Inc., Chicago, IL, USA). The PFS was calculated as time from the date of treatment to the first recurrence of disease at local, regional, or distant site or death. The OS ended with the date of tumor-associated death. The Kaplan-Meier method and the log-rank test were used to analyze potential prognostic factors. P < 0.05 was considered statistically significant. The Cox proportional hazards regression model was used for the analyses taking into account all variables simultaneously.
Results
Patient characteristics
All 90 patients received at least one dose of trastuzumab, but 5 patients only received one dose of trastuzumab for observing safety profile only. The remaining 85 patients could be evaluated for efficacy and safety. The duration of follow-up was from January 2006 to September 2014, and the median followup duration was 50 months (range, 8-352 months).
This cohort had more aggressive disease and only 9 patients received trastuzumab in an adjuvant setting. In total, 37.78% of cases were hormone receptor negative, 59.26% had lymph node metastasis at initial diagnosis after surgery, and 9 patients had initial advanced disease with multiple site metastasis. Twenty-nine (32.22%) patients were diagnosed with lung metastasis and 56 (62.22%) with liver metastasis, while 80.00% had invasive metastasis (both lung and liver involvement), and 3 or more metastatic sites were evident in 35.56% of patients. Furthermore, 65.43% (53/81) of patients experienced a relapse within 3 years, and 77 (85.56%) patients had already undergone adjuvant chemotherapy with anthracycline and/or taxane. At the time of trastuzumab treatment, 43 (47.78%) patients had progressed after one or more extensive prior palliative chemotherapy regimens for a metastatic setting, 4 had initial advanced disease; 20 as second-line, 15 as third-line, and 8 for beyond third-line treatment. The basic patient characteristics are shown in Table 1.
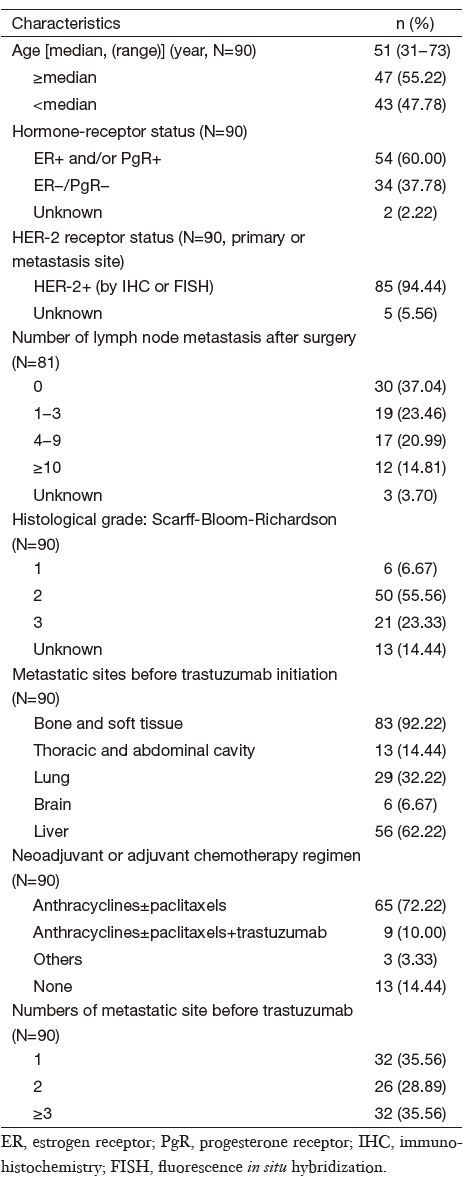
Full table
Treatment
All 90 patients were treated with trastuzumab combined with chemotherapy. The first-line or subsequent lines of trastuzumab was used as the initial anti-HER2 therapeutic agent in different patient settings. There were 47 patients received trastuzumab as first-line therapy, after progression from first-line trastuzumab treatment, the number of patients who continued trastuzumab treatment as second-line, thirdline and beyond therapy were 34,14, and 6, respectively. In total, there were 920 treatment cycles in this group of patients, the median number was 8 cycles (range, 2-70 cycles), and 49 (54.4%) patients received trastuzumab for more than 8 cycles. For second-line treatment, only 3 patients were treated longer for more than 1 year, with 15,18, and 18 cycles, respectively. For third-line treatment, only 3 of 14 patients were treated with more than 5 cycles (2 patients underwent 5 cycles and 1 patient underwent 8 cycles), while for fourth-line treatment and beyond the median number of cycles, it was very low at 3 cycles (range, 2-5 cycles).
For chemotherapy agents, at first-line treatment, 56 patients received paclitaxel/docetaxel, paclitaxel was given 175 mg/m2 in the first day (d 1), or 175 mg/m2 divided in d 1 and the eighth day (d 8), every 3 weeks (q3w), and docetaxel was given 75 mg/ m2, d 1, q3w; 19 patients received vinorelbine (30 mg/m2, d 1and d 8, q3w); 11 received gemcitabine (1,000 mg/m2, d 1 and d 8, q3w); and 27 patients received capecitabine [1,000 mg/ m2, d 1-d 14, twice per day (bid)]. The most frequently used combination regimens were taxane plus platinum in 12 patients, paclitaxel/docetaxel plus capecitabine (TX) in 9 patients, paclitaxel/docetaxel plus gemcitabine (TG) in 5 patients, and vinorelbine plus capecitabine (NX) in 7 patients. In the secondline and third-line setting, chemotherapy agents were different from first-line agents. We also analyzed 44 patients treated with single agent chemotherapy and compared with 41 patients treated with combination chemotherapy.
Treatment efficacy
A total of 85 patients could be evaluated for efficacy of first-line trastuzumab in advanced disease, resulting in a median PFS of 10 months (range, 2-59 months), a median OS from the date of trastuzumab treatment to the date of death from any cause of 16 months (range, 2-70 months), and a median OS after metastasis or initially local advanced disease of 22 months (range, 2-116 months). The median OS from initially diagnoses was 50 months (range, 8-352 months) (Table 2).
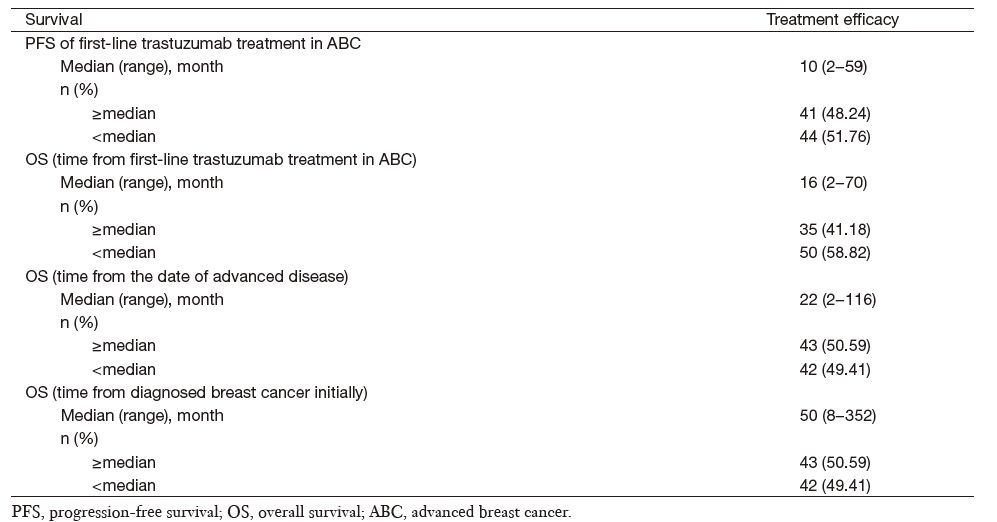
Full table
Complete response (CR) in first-line trastuzumab treatment was achieved in 5/85 (5.9%) patients, and partial response (PR) in 37/85 (43.5%), while 28/85 (32.9%) experienced stable disease (SD). This resulted in an ORR of 49.4% (Table 3).
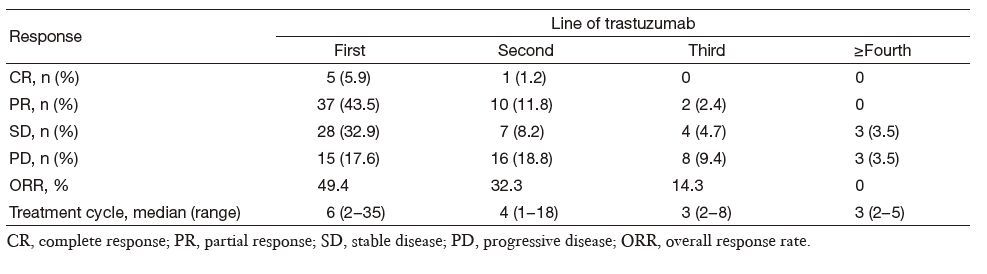
Full table
Prognostic factors for activity of trastuzumab
The impact of several prognostic characteristics on ORR and PFS was analyzed, focusing on the subgroups of patients continuing trastuzumab treatment after first-line trastuzumab therapy, or those who received trastuzumab in different disease conditions, and/or those who were administered trastuzumab in parallel with a single or combination chemotherapy agent.
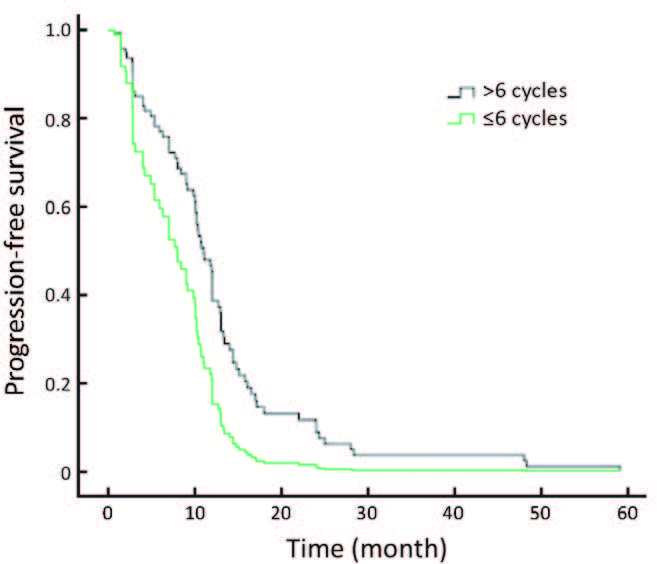
The efficacy for continuing trastuzumab treatment after disease progression and trastuzumab in different disease statuses really influenced the results. Patients who progressed after their initial trastuzumab treatment continued to receive trastuzumab, and their response rates are shown in Table 3. Among 34,14 and 6 patients who continued using trastuzumab in the second-line, third-line and beyond fourth-line settings, respectively, 1 achieved CR, 10 achieved PR in the second-line, and 2 achieved PR in the third-line. While no ORR was obtained after this point, longer therapeutic cycles were employed in the early lines of trastuzumab treatment (Table 3), with concomitant longer PFS. Patients with different therapeutic cycles (>6 cycles vs. 6 cycles) showed statistically different PFS (P = 0.007; Figure 1).
Trastuzumab was used as the initial anti-HER2 therapeutic agent in different patients. There were 47/85 (55.3%) patients treated with trastuzumab in the first relapse disease. The ORR (CR+PR) for disease was 57.4%, with a CR of 10.6% (5/47) and a PR of 46.8% (22/47). After that, the ORR was lower. The trastuzumab treatment achieved high responses in patients without previous chemotherapy for advanced disease, and better PFS was obtained compared with later disease treatment (P=0.004; Figure 2).
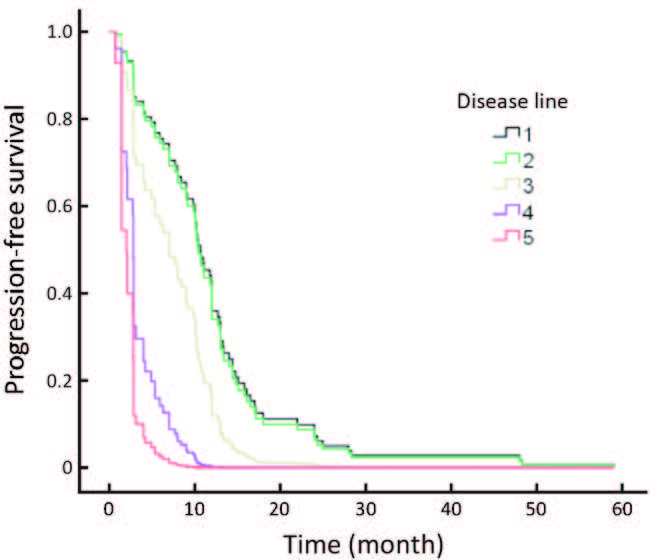
No major prognostic impact was detected for hormone receptor status or age, or for other factors that may influence efficacy such as the site of metastasis or the number of metastatic sites. Because in this cohort, 80.00% of patients had liver and lung metastasis, and 64.44% had more than two sitesof metastasis. Different chemotherapy agents were not analyzed in this study, because 62.22% of patients underwent taxanebased chemotherapy, and thus, this population subgroup was imbalanced.
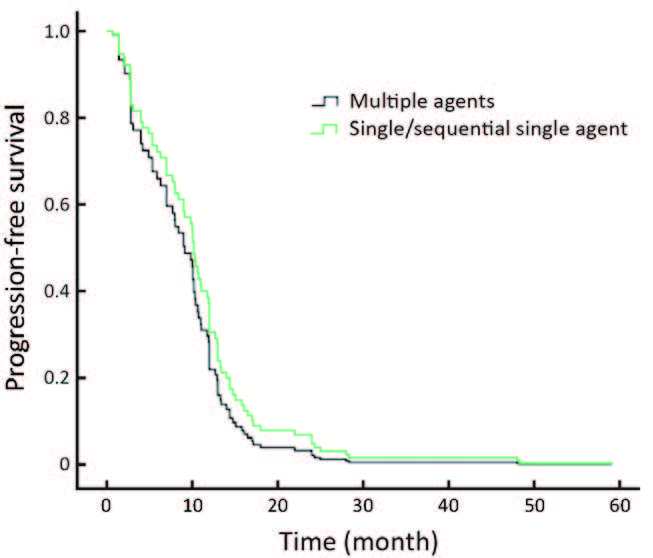
Regarding the chemotherapy regimen, the PFS of patients undergoing single agent chemotherapy was not statistically different compared with those receiving combination chemotherapy treatment (P=0.305; Figure 3).
Safety profile
A safety analysis was performed in all 90 patients who received at least one dose of trastuzumab, however, most patients were treated with chemotherapy simultaneously so chemotherapyrelated side effects such as hemotological effects are not presented here. Nine patients ceased treatment because of the following side effects: angina pectoris in 3 patients (1 of whom stopped treatment immediately), liver failure (multiple liver metastasis) in 1, lung injury in 2, serum creatinine increase in 1, thrombus in 1, and finger necrosis in 1. Furthermore, 8 patients refused further treatment because of high costs, and 15 were diagnosed with brain metastases during the treatment period. The other most common treatment-related adverse events were chill in 11 (12.2%) patients, and fever in 15 (16.6%). Most treatment-related adverse events were mild to moderate, except in 5 patients who stopped treatment immediately, and most occurred during the first drug infusion.
Discussion
Trastuzumab in combination with chemotherapy is the standard of care for HER2-positive breast cancer. However, the most widely-used treatment for advanced breast cancer in China is trastuzumab as a result of a public welfare project in 2011. Thus, there remains the need to find new combinations or new schedules to prolong clinical benefit and survival of this subset of patients. In this observational study, the majority of the cohort of patients was enrolled over the past 2 years. We evaluated the use of trastuzumab in advanced HER2-positive breast cancer, and our data represent important information on the application, efficacy, and safety of trastuzumab in clinical practice.
Our study found that high response rate was more frequently attained in earlier therapy, like disease in the firstline CR achieved in 5/85 (5.8%) patients, but only one CR in the second-line setting. That means we should consider treating patients by trastuzumab as early as possible. And besides, in this cohort, there was a high proportion of patients with aggressive diseases coupled with liver and lung metastasis, more multiple sites of metastasis and more previous rounds of chemotherapy for advanced disease, but we still can see the response and prolonged patients survival. Therefore, patients can be treated in some aggressive situations.
In a report from France, of 1,234 patients with metastatic breast cancer treated with chemotherapy between 2001 and 2010,217 patients received trastuzumab. In this subset, 38% had liver metastases at first occurrence of advanced breast cancer, and the median OS was 45.2 months (15). Another phase Ⅱ Trial (M77001) (16) of first-line trastuzumab with docetaxel demonstrated an ORR of 61% [95% confidence interval (95% CI),50%-71%],6 CRs (7%) and 50 PRs (54%), with stable disease observed in 27%. This result also indicated that trastuzumab had a high response as a first-line treatment (17). The patients who continued trastuzumab beyond disease progression achieved modest responses (18), as observed in an original pivotal trial (also an extension study), in which 247 patients were enrolled and the ORR was 11% (19).
Additionally, our data also showed that patients receiving two or more trastuzumab-containing regimens survived significantly longer than those who discontinued trastuzumab in disease progression. Here we presented a PFS that was significantly longer following more than six cycles of trastuzumab treatment (9 months vs. 16 months; P=0.007).
This is especially inspiring under the current circumstances since pertuzumab and T-DM1 are not available in China for metastatic breast cancer patients as alternative options foranti-HER2 treatment. Therefore, trastuzumab could be a maintenance therapy for these patients, especially in first to third lines.
An Italian retrospective analysis identified a trend for better survival from the start of trastuzumab treatment in patients continuing beyond disease progression compared with those who halted the drug [hazard ratio (HR)=0.78; 95% CI: 0.58-1.32] (20), although the response rate tended to be lower with each subsequent line. In this trial, ORR in the first-line, secondline, third-line and fourth-line setting was 35%,16%,15%, and 0%, respectively, which is similar to our data.
However, in the GBG 26/BIG 3-05 phase Ⅲ study (21), a significant survival benefit for treatment beyond progression with trastuzumab was not demonstrated. Nevertheless, another retrospective study reported no clinical benefit from continuing trastuzumab beyond progression in metastatic breast cancer (22). In this study, vinorelbine-based salvage therapies were retrospectively investigated in 60 patients progressing during initial treatment with a trastuzumab-based regimen. Twentynine patients receiving vinorelbine-based salvage treatment and continuing trastuzumab had an ORR of 21%. In the 31 patients who stopped trastuzumab, the ORR was 36%. These data imply that the efficacy of trastuzumab would continue across multiple lines of therapy, and it also had limitations. However, our results also showed that there is a difference between early trastuzumab treatments compared with later usage. These findings revealed that the ORR became sequentially worse though disease progression from the first-, second-, thirdline setting and beyond at 31.8%,10.6%,7.1%, and 0%, respectively. A similar study reported that women with HER2- overexpressing metastatic breast cancer had progressed after one or two chemotherapy regimens, 8 achieved CR and 26 achieved PR, with an ORR of 15% in the intent-to-treat population. The median duration of response was 9.1 months, and the median duration of survival was 13 months (23). This also suggested that anti-HER2 therapy should be used as early as possible.
The most frequently used chemotherapy agents were paclitaxel, docetaxel or vinorelbine, gemcitabine and even carboplatin (3, 24). There is no evidence to indicate a preferred chemotherapy regimen in the first-line setting for advanced breast cancer treatment (24). In the above mentioned German study (3), the ORR was the highest in the subgroup receiving trastuzumab together with chemotherapy (60%). Our study found that in HER2-positive patients, a taxane-based regimen presented a better PFS but not OS benefit compared with nontaxane- based regimens when combined with trastuzumab. We also found vinorelbine or gemcitabine obtained activity with trastuzumab in our study. However, a relatively high proportion of patients who received taxane were included in our study, which could bias the results. This implies that all of the described drugs should be considered as alternative firstline options.
Another interesting result found in our study was that there was no difference in PFS between patients treated with either single/sequential-single chemotherapy or combined chemotherapy. This observation was also showed in the BCIRG 007 Study (25), which presented a response rate of 72% for both docetaxel plus trastuzumab (TH) and docetaxel plus carboplatin and trastuzumab (TCH) groups’ treatment regimens, and median OS of 37.1 and 37.4 months, respectively. This differed from another study, which showed that docetaxel plus capecitabine and trastuzumab (TXH) treatment demonstrated significantly longer PFS, with a median of 17.9 months compared with 12.8 months with TH (26), which translates to a gain of around 5 months. These findings suggested that for metastatic breast cancer, single/sequential-single chemotherapy rather than combined chemotherapy should be considered to attain survival benefit and a better quality of life for patients.
No additional adverse events were observed in our study compared with previous results, for example, cardiotoxicity was evident in 3/90 (3%) patients in our study, which was similar to a report of single agent use of trastuzumab in which 2% of patients exhibited cardiotoxicity (27). The most common treatment-related adverse events were chill and fever, which were similar to another published result (28).
Conclusions
In conclusion, trastuzumab combined with chemotherapy was active and well tolerated as a first-line treatment for patients with HER2-overexpressing advanced breast cancer. Even for patients who progressed upon trastuzumab treatment, continued trastuzumab treatment still showed efficacy but not as good as that observed for first-line therapy. Earlier administration and longer duration of trastuzumab treatment tended to have more clinical benefits for advanced breast cancer patients. Furthermore, trastuzumab combined with a single chemotherapy agent delivered similar efficacy compared with combined chemotherapy, and should be considered as an option to achieve survival benefit and a better quality of life for patients.
Acknowledgements
We gratefully acknowledge the independent assessment of tumor response by radiologists, and the contribution of all of the women who were treated in this observation study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 1989;244 :707–12. [PubMed]
- Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol, 2014;21 :100–107. [PubMed]
- Jackisch C, Schoenegg W, Reichert D, et al. Trastuzumab in advanced breast cancer--a decade of experience in Germany. BMC Cancer, 2014;14 :924. [PubMed]
- De Mattos-Arruda L, Cortes J. Advances in first-line treatment for patients with HER-2+ metastatic breast cancer. Oncologist, 2012;17 :631–644. [PubMed]
- Mendes D, Alves C, Afonso N, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res, 2015;17 :140. [PubMed]
- Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase Ⅲ study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol, 2006;24 :2786–2792. [PubMed]
- Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase Ⅲ TAn DEM study. J Clin Oncol, 2009;27 :5529–5537. [PubMed]
- Ahmed S, Sami A, Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer, 2015;22 :101–116. [PubMed]
- Von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol, 2009;27 :1999–2006. [PubMed]
- Sun Y, Li LQ, Song ST, et al. Result of phase Ⅱ clinical trial on herceptin in advanced Chinese breast cancer patients. Zhonghua Zhong Liu Za Zhi (in Chinese), 2003;25 :581–583.
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast, 2014;23 :489–502. [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol, 2014;25 :1871–1888. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada.. J Natl Cancer Inst, 2000;92 :205–216. [PubMed]
- Common Terminology Criteria for Adverse Events version 3.0 (CTCAE). Available online: http://119.90.25.41/ctep. cancer.gov/protocolDevelopment/electronic_applications/ docs/ctcaev3.pdf
- Fiteni F, Villanueva C, Bazan F, et al. Long-term followup of patients with metastatic breast cancer treated by trastuzumab: impact of institutions. Breast, 2014;23 :165–169. [PubMed]
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase Ⅱ trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol, 2005;23 :4265–4274. [PubMed]
- Yeo B, Kotsori K, Mohammed K, et al. Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast, 2015;24 :751–757. [PubMed]
- Rayson D, Lutes S, Walsh G, et al. Trastuzumab beyond progression for HER2 positive metastatic breast cancer: progression-free survival on first-line therapy predicts overall survival impact. Breast J, 2014;20 :408–413. [PubMed]
- Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol, 2004;22 :1063–1070. [PubMed]
- Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer, 2004;5 :52–58. [PubMed]
- von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase Ⅲ study in HER2- positive breast cancer. Eur J Cancer, 2011;47 :2273–2281. [PubMed]
- Montemurro F, Redana S, Nolè F, et al. Vinorelbinebased salvage therapy in HER2-positive metastatic breast cancer patients progressing during trastuzumab containing regimens: a retrospective study. BMC Cancer, 2008;8 :209. [PubMed]
- Cobleigh MA, Vogel CL, Tripathy, et al. Multinational study of the efficacy and safety of humanized anti- HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol, 1999;17 :2639–2648. [PubMed]
- Andersson M, Lidbrink E, Bjerre K, et al. Phase Ⅲ randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol, 2011;29 :264–271. [PubMed]
- Valero V, Forbes J, Pegram MD, et al. Multicenter phase Ⅲ randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol, 2011;29 :149–156. [PubMed]
- Wardley AM, Pivot X, Morales-Vasquez F, et al. Randomized phase Ⅱ trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol, 2010;28 :976–83. [PubMed]
- Rossi M, Carioli G, Bonifazi M, et al. Trastuzumab for HER2+ metastatic breast cancer in clinical practice: Cardiotoxicity and overall survival. Eur J Cancer, 2016;52 :41–49. [PubMed]
- Adamo V, Franchina T, Adamo B, et al. Safety and activity of trastuzumab-containing therapies for the treatment of metastatic breast cancer: our long-term clinical experience (GOIM study). Ann Oncol, 2007;18 :vi11–5.
