Expanded endoscopic therapy criteria should be cautiously used in intramucosal gastric cancer
Hongshan Wang*, Heng Zhang*, Cong Wang, Yong Fang, Xuefei Wang, Weidong Chen, Fenglin Liu, Kuntang Shen, Xinyu Qin, Zhenbin Shen, Yihong Sun
*These authors contributed equally to this study.
Abstract
Objective: Accurate estimation of lymph node metastasis (LNM) in intramucosal gastric cancer is essential to select less invasive treatment options and even avoid surgery. The aim of this study was to evaluate combined clinicopathological features to predict the presence of LNM.
Methods: A retrospective review of data from 386 intramucosal gastric cancer patients who underwent gastrectomy with extended lymphadenectomy from 2003 to 2010 was conducted. The mutual relation between clinicopathological characteristics and LNM was analyzed.
Results: LNM was detected in 40 (10.4%) of the 386 patients. Histological type and vascular or lymphatic invasion presence showed a positive correlation with LNM occurrence by univariate analysis. Multivariate analysis revealed that histological type was the only factor associated with LNM. Combined clinicopathologic characteristics would be more predictable for LNM. We found no LNM when we used combined clinicopathological characteristics conforming to Japanese absolute indications for endoscopic therapy. The LNM rate was as high as 8.7% when Japanese expanded criteria were used. Univariate analysis in cancer conformity to expand endoscopic submucosal dissection (ESD) indication also revealed that the undifferential type was the only significant factor for LNM.
Conclusions: It was possible to predict intramucosal gastric cancer cases without LNM using combined clinicopathological characteristic analysis. Extended indication for ESD should be cautiously used for intramucosal gastric cancer patients.
Keywords: Lymph node metastasis; early gastric cancer; intramucosal cancer; endoscopic therapy
Submitted Jan 13, 2015. Accepted for publication Mar 22, 2016.
doi: 10.21147/j.issn.1000-9604.2016.03.09
Introduction
From GLOBOCAN2012 (http://globocan.iarc.fr/), gastric cancer is the fourth most common malignancy in the world, the third leading cause of cancer death in males, and the fifth leading cause of death in females. The high incidence and high mortality of gastric cancer were also been reported in China (1). Surgery is the standard treatment for early gastric cancer (EGC) in most countries. Surgical resections are designed to remove not only the gastric lesion but also the potentially involved regional lymph nodes. Data from Eastern Asia showed that the EGC recurrence rate after gastrectomy was very low, varying from 1.3% to 2.2% (2-4). However, gastrectomy with lymphadenectomy carries the risks of complications and perioperative death. Patients with severe co-morbidities are at high risks. For the general population, surgical intervention could cause functional and symptomatic problems and impair quality of life (QOL) (5, 6). Since most intramucosal gastric cancers do not have lymph node metastasis (LNM) (7-9), unnecessary extended surgery could be avoided in patients with EGC without LNM. Thus, endoscopic therapy such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) may provide an attractive and less invasive treatment option that may be safe and effective in a selected patient subgroup.
Many studies on EGC have shown indications for minimally invasive surgery without lymph node dissection (10, 11). Multivariate analyses performed on large patient groups in Japan showed that lymph node involvement incidence would be less than 0.4% if some criteria were met (12). The resection is judged as curative when all of the following conditions are fulfilled: en-bloc resection, tumor size ≤2 cm, histologically differentiated-type, pT1a, negative horizontal margin (HM0), negative vertical margin (VM0), and no lymphovascular infiltration (ly(-), v(-)) (13). The prognostic analyses demonstrated the endoscopic therapy criteria’s validity (14). ESD under expanded criteria is still investigational treatments (T1a and: a) of differentiated-type, UL (-), but >2 cm in diameter; b) of differentiated-type, UL (+), and ≤3 cm in diameter; or c) of undifferentiated-type, UL (-), and ≤2 cm in diameter) (12) and more evidence is needed to prove expanded criteria’s safety.
Apart from Japan, endoscopic technique is increasingly gaining acceptance (15, 16). Clinicopathological characteristics of intramucosal gastric cancer associated with LNM, which may be diverse in patients from different regions, should also be extensively investigated when applying endoscopic treatment for EGC to other areas. Few reports have been published outside of Japan and Korea (17, 18). This study investigated the clinicopathological characteristics of intramucosal gastric cancer to identify LNM-associated factors and discuss if Japanese practice is suitable for patients outside of Japan and Korea.
Methods
Patients
Intramucosal gastric cancer is defined as the lesion confined in mucosa without penetrating through muscularis mucosa. This study enrolled 386 intramucosal gastric cancer patients who underwent R0 resection as an initial treatment in Zhongshan Hospital, Shanghai, China between 2003 and 2010. The operative procedures include gastrectomy with sufficient margin and extensive lymphadenectomy. Patients who previously underwent upper gastrointestinal surgery or endoscopic treatment were excluded. Gastric carcinomas were classified using the Japanese Classification of Gastric Carcinoma (19). All resected specimens were pathologically diagnosed as intramucosal cancer.
Clinicopathological data
We retrieved demographic data (age and gender) and tumor information (size, stomach location, macroscopic type, ulcer presence, histological classification, and the presence of vascular or lymphatic invasion) from medical documents. The differentiated group included papillary adenocarcinoma and well or moderately differentiated tubular adenocarcinoma, and the undifferentiated group included poorly differentiated or undifferentiated adenocarcinoma, mucinous, and signet ring cell carcinoma. We measured tumor size by the longest diameter and confirmed ulcer presence in formalin-fixed tissue based on the gross or histological findings from the resected specimen.
Postoperative treatment and follow-up
Moreover, EGC patients that presented with LNM would be recommended for adjuvant chemotherapy. However, only 42.5% of EGC patients with LNM received adjuvant chemotherapy. The standard follow-up protocol included physical examination, gastroscopy, chest X ray, abdominal enhanced computed tomography (CT) scan, serum carcinoembryonic antigen (CEA), and analysis of CA19-9 every 6-12 months.
Statistical analysis
The association of LNM with clinicopathological variables was assessed using Fisher’s exact test. Factors found to be significant by univariate analysis were included in subsequent multivariate logistic regression analysis to identify those variables independently associated with LNM. Overall survival curve analysis was estimated by the Kaplan-Meier method and compared by the log-rank test that was stratified by lymph node status. P < 0.05 was considered to be statistically significant. The probability of LNM was estimated with 95% confidence interval (95% CI) based on binominal distribution. All statistical analyses were conducted using SPSS statistical program (version 19; SPSS Inc., Chicago, IL, USA).
Results
Patient clinical characteristics
Between 2003 and 2010,712 gastric cancer patients were confirmed as EGC with complete surgical information and pathological data. Of these patients, 386 (246 males, 140 females) intramucosal cancer cases were enrolled in this study. Their average age was 51.7 years old (range, 33-85 years). Their standard operative procedures include distal gastrectomy (329 cases), proximal gastrectomy (10 cases), or total gastrectomy (47 cases) depending on the tumor location with at least 1 cm clear margin and D1+ (151 cases) or D2 dissection (235 cases). The mean number of retrieved lymph nodes was 22.3 (range, 11-43).
Relationship between clinicopathological features and LNM in intramucosal gastric cancer patients
We observed 40 LNM cases (10.4%) out of 386 intramucosal gastric cancer cases. A univariate analysis of the clinicopathological characteristics for LNM showed histological type and vascular or lymphatic invasion presence to be significant factors. The LNM rate in patients with well differentiated cancer was 3.1% (5/160), respectively lower than 15.4% (35/226) that in the poorly differentiated or undifferentiated group (P < 0.001). The LNM rate classified by vascular or lymphatic involvement was 50% (3/6) with involvement and 9.7% (37/380) without involvement (P = 0.001) (Table 1). According to multivariate analysis, the histological type was the only statistically significant variable (odds ratio (OR)=3.852; 95% CI, 1.658-8.948; P = 0.002) while vascular or lymphatic involvement was not confirmed as significant factor (Table 2).
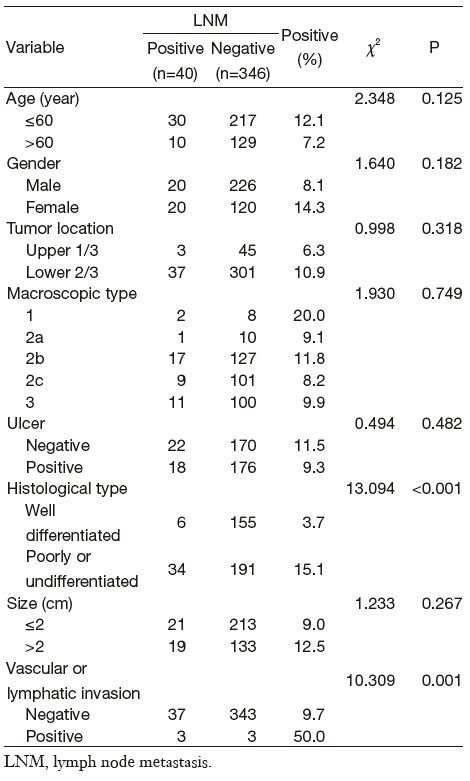
Full table
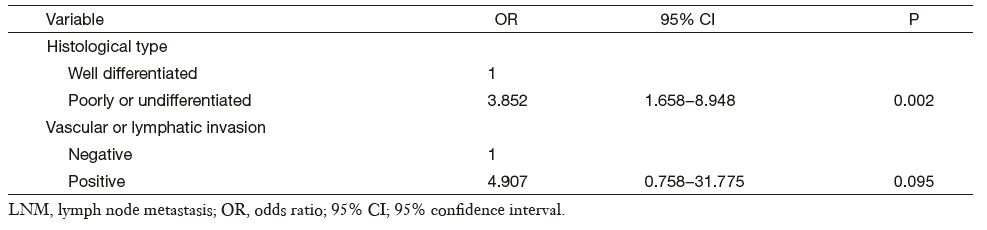
Full table
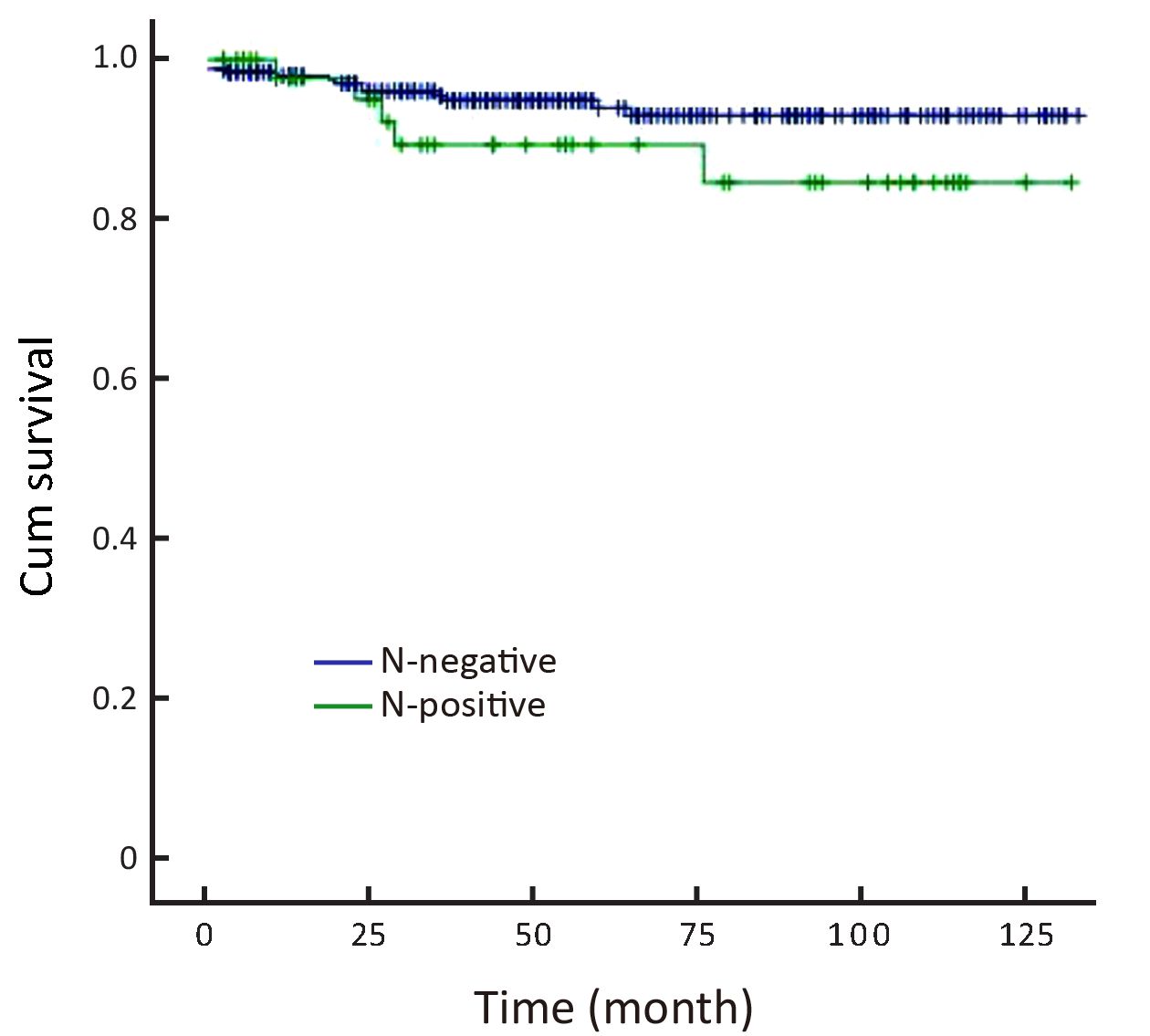
Combined clinicopathological characteristics would be more predictable for LNM. Absolute indication for endoscopic therapy in Japanese gastric cancer treatment guidelines (version 3) would predict cases without LNM in our series, but such cases were relatively rare (only 10.4%). When we used expanded indication, the LNM rate increased to as high as 8.7% (16/183) (Table 3). Univariate analysis of potential risk factors (tumor size, ulcer presence, histological type, and vascular or lymphatic invasion) in intramucosal gastric cancer conforming to expanded ESD indication also showed that the undifferential type was the only significant factor for LNM (Table 4). Additionally, the 5-year overall survival for this cohort was 93.3% (N-negative 93.9% and N-positive 84.6%, P = 0.161, Figure 1).
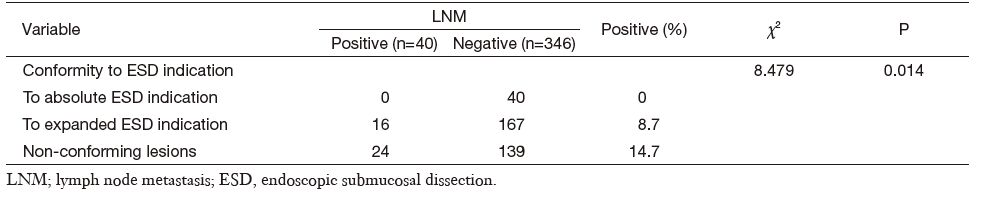
Full table
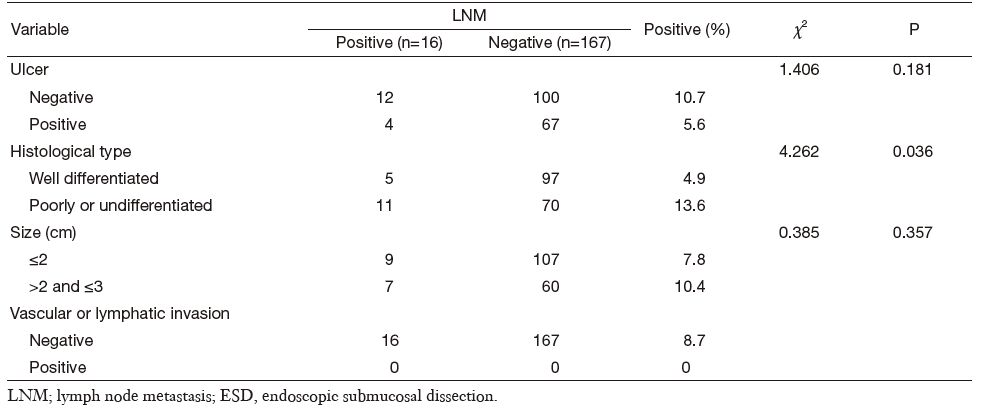
Full table
Discussion
With the increasing of gastric cancer screening, the proportion of EGC cases increases. The incidence of nodal involvement for EGC is very low in the Japanese literature and ranges from 1.9% to 4.8% for intramucosal cancers and 16.5% to 23.6% for submucosal cancers (7-9). This study demonstrated lymph node involvement rates of 10.4% for intramucosal cancers. Since we performed endoscopic resection for 64 intramucosal cancer cases with very low LNM risk in Zhongshan Hospital during the study period, LNM incidence could be 8.9% if endoscopically treated cases were included. The LNM incidence was similar to data reported by Huang (6.4%) (20), Feng (8.3%) (21) and Degiuli (10.8%) (22). There are several differences in diagnostic criterion between Western and Japanese pathologists (23). Japanese pathologists are less strict in diagnosing gastric cancer than their Western colleagues. Different histological criteria between China and Japan could be another reason for the slightly higher LNM incidence in our series. Some intestinal type intramucosal cancers in Japanese criterion may be diagnosed as high-grade dysplasia and were excluded from analysis in this study. The relatively lower intramucosal cancer LNM incidence in Japan makes it more reasonable to choose treatment modalities other than surgery. Only those without LNM were definitely suitable for endoscopic therapy.
Deciding how to screen out intramucosal gastric cancer patients without LNM is still a controversial issue. Accurate evaluation of LNM status is essential for choosing less invasive therapy. Imaging techniques like CT, multi-detector CT (MDCT), or endoscopic ultrasonography (EUS) are unable to detect metastatic lymph nodes precisely, leaving the risk of under-treatment when choosing endoscopic methods. Clinicopathological factors are helpful in predicting LNM. Tumor size, invasion depth, gross appearance, histological type, vascular invasion or lymphatic involvement and perineural involvement have been demonstrated as related to LNM in EGC (8, 12, 24-26). For intramucosal gastric cancer, Yuasa reported tumor size to be the major LNM risk factor (27). In our study, univariate analysis showed the presence of histological type and vascular or lymphatic invasion to be significant factors, and multivariate analysis revealed histological type was the only factor associated with LNM. The prognostic vascular or lymphatic invasion features still could not be denied. Precise pathological biopsy specimen diagnosis with necessary information is important when choosing endoscopic therapy candidates. The diagnosis of "mucosal high grade neoplasia", which comprises a continuum extending from high-grade dysplasia to intramucosal gastric cancer according to the Vienna classification of gastrointestinal epithelial neoplasia (28, 29), would be confusing when choosing endoscopic treatment since the term would not provide necessary information needed to evaluate LNM.
There is no single clinicopathological factor that can predict LNM independently. Combined clinicopathological characteristics may screen out patients without LNM. In our series, 40 cases matched Japanese absolute indication for endoscopic therapy had no LNM, which would support the rationality of these criteria. Expanded indication is also listed in Japanese guidelines for further research. According to expanded criteria, our data showed that the LNM rate was as high as 8.7%, suggesting endoscopic treatment should be cautiously selected in these patients. Hirasawa found that those of the undifferentiated type with an intramucosal EGC 20 mm or less in size without lymphatic-vascular capillary involvement, and ulcerative findings presented a negligible LNM risk (30). However, the undifferentiated type was the only significant factor for LNM in our series, and the endoscopic treatment is not suitable for these patients. Undifferentiated lesions associated with significant metastasis were reported when radical gastrectomy was performed after ESD (31, 32). Some other cases with regional lymph node relapse shortly after ESD was reported (33).
This study had some limitations. Since the gross appearance of formalin-fixed specimens is different from how they looked under endoscopy observation, there is a limitation in predicting lymph node metastatic disease with a formalin-fixed specimen. Song from Korea also doubted the retrospective analysis of pathological reports. He found that histological indicators seem to predict lymph node metastatic disease in limited surgical specimens (24). Detailed endoscopic reports would provide the most accurate description of a gastric lesion’s gross appearance and should be combined with surgical information and pathological data in prospective studies in the future.
Conclusions
Predicting intramucosal gastric cancer cases without LNM was possible with combined clinicopathological characteristics analysis. Japanese extended indication for ESD should be cautiously used for Chinese intramucosal gastric cancer patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res, 2015;27 :2–12. [PubMed]
- Youn HG, An JY, Choi MG, et al. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol, 2010;17 :448–54. [PubMed]
- Gunji Y, Suzuki T, Hori S, et al. Prognostic significance of the number of metastatic lymph nodes in early gastric cancer. Digest Surg, 2003;20 :148–53.
- Sano T, Sasako M, Kinoshita T, et al. Recurrence of early gastric cancer. Follow-up of 1,475 patients and review of the Japanese literature. Cancer, 1993;72 :3174–3178. [PubMed]
- Kurokawa Y, Sasako M, Sano T, et al. Functional outcomes after extended surgery for gastric cancer. Br J Surg, 2011;98 :239–45. [PubMed]
- Kim AR, Cho J, Hsu YJ, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg, 2012;256 :1008–13. [PubMed]
- Pelz J, Merkel S, Horbach T, et al. Determination of nodal status and treatment in early gastric cancer. Eur J Surg Oncol, 2004;30 :935–41. [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer, 2000;3 :219–25. [PubMed]
- Haruta H, Hosoya Y, Sakuma K, et al. Clinicopathological study of lymph-node metastasis in 1,389 patients with early gastric cancer: assessment of indications for endoscopic resection. J Digest Dis, 2008;9 :213–8.
- Son SY, Park JY, Ryu KW, et al. The risk factors for lymph node metastasis in early gastric cancer patients who underwent endoscopic resection: is the minimal lymph node dissection applicable? A retrospective study. Surg Endosc, 2013;27 :3247–53. [PubMed]
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery, 1999;125 :148–54. [PubMed]
- Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer, 1996;77 :602–6. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer, 2011;14 :113–123. [PubMed]
- Goto O, Fujishiro M, Kodashima S, et al. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy, 2009;41 :118–22. [PubMed]
- Rembacken BJ, Gotoda T, Fujii T, et al. Endoscopic mucosal resection. Endoscopy, 2001;33 :709–18. [PubMed]
- Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc, 2003;57 :567–79. [PubMed]
- Schumacher B, Charton JP, Nordmann T, et al. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc, 2012;75 :1166–74. [PubMed]
- Rushfeldt C, Aabakken L. Implementation of endoscopic submucosal dissection for gastric lesions in Norway. Scand J Surg, 2016;105 :90–6. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer, 2011;14 :101–12. [PubMed]
- Huang BJ, Lu C, Xu YY, et al. Study on lymph node metastasis in 292 patients with early gastric cancer. Zhonghua Wai Ke Za Zhi, 2007;45 :192–195. [PubMed]
- Feng F, Sun L, Xu G, et al. Is it reasonable to treat early gastric cancer with mucosal infiltration and well differentiation by endoscopic submucosal resection?. J Gastrointest Surg, 2015;19 :2111–2119. [PubMed]
- Degiuli M, Calvo F. Survival of early gastric cancer in a specialized European center. Which lymphadenectomy is necessary? World J Surg, 2006;30 :2193–203. [PubMed]
- Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch, 2003;442 :99–106. [PubMed]
- Kim HS, Lee DK, Baik SK, et al. Endoscopic mucosal resection with a ligation device for early gastric cancer and precancerous lesions: comparison of its therapeutic efficacy with surgical resection. Yonsei Med J, 2000;41 :577–583. [PubMed]
- Song SY, Park S, Kim S, et al. Characteristics of intramucosal gastric carcinoma with lymph node metastatic disease. Histopathology, 2004;44 :437–44. [PubMed]
- Zheng Z, Liu Y, Bu Z, et al. Prognostic role of lymph node metastasis in early gastric cancer. Chin J Cancer Res, 2014;26 :192–9. [PubMed]
- Yuasa N, Nimura Y. Survival after surgical treatment of early gastric cancer, surgical techniques, and long-term survival. Langenbecks Arch Surg, 2005;390 :286–93. [PubMed]
- Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut, 2002;51 :130–1. [PubMed]
- Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut, 2000;47 :251–5. [PubMed]
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer, 2009;12 :148–52. [PubMed]
- Chung JW, Jung HY, Choi KD, et al. Extended indication of endoscopic resection for mucosal early gastric cancer: analysis of a single center experience. J Gastroenterol Hepatol, 2011;26 :884–7. [PubMed]
- Toyokawa T, Ohira M, Tanaka H, et al. Optimal management for patients not meeting the inclusion criteria after endoscopic submucosal dissection for gastric cancer. Surg Endosc, 2016;30 :2404–14. [PubMed]
- Nagano H, Ohyama S, Fukunaga T, et al. Two rare cases of node-positive differentiated gastric cancer despite their infiltration to sm1, their small size, and lack of lymphatic invasion into the submucosal layer. Gastric Cancer, 2008;11 :53–7. [PubMed]
