Making cancer fat: reprogramming of lipid metabolism by CD147 in hepatocellular carcinoma
Hye-Lim Ju, Simon Weonsang Ro
Submitted Jan 18, 2016. Accepted for publication Feb 19, 2016.
doi: 10.21147/j.issn.1000-9604.2016.03.14
Over the past few years, de novo lipogenesis has taken central stage in the field of cancer metabolism (1). Large amount of lipids is needed for synthesis of membranes, signaling molecules, lipoproteins, etc. to support rapidly growing tumor cells (2-4). Reports have shown that neoplastic tissues show aberrant activation of de novo lipogenesis and that inhibition of different enzymes within the fatty acid biosynthesis pathway can block cancer cell growth (2, 5-9). Meanwhile, the importance of fatty acid oxidation (FAO) in cancer metabolism is being increasingly recognized. FAO is the catabolic process by which lipids are utilized to produce energy. Recent studies implicated that the key regulatory enzymes in FAO such as carnitine palmitoyltransferase 1 (CPT1) and peroxisomal acyl-coenzyme A oxidase 1 (ACOX1) regulate cancer development (10, 11). The underlying mechanisms for the regulation of de novo lipogenesis and FAO in cancers are, however, still incompletely understood. Thus, it would be of a high scientific and clinical interest to elucidate the lipid metabolism in cancer.
Multiple independent laboratories discovered that CD147, a transmembrane glycoprotein, is highly expressed in hepatocellular carcinoma (HCC) cells and is strongly associated with tumor progression (12, 13). Licartin, an 131Iodin-labeled antibody fragment targeting the HCCassociated antigen HAb18G/CD147, has been approved by the Chinese Food and Drug Administration (FDA) and enters into clinical use for HCC treatment (14-16). To date, studies have shown that CD147 contributes to the metabolism of cancer cells via glycolysis (17-19). However, a paper recently published in the Journal of Hepatology by Li et al. reports that CD147 regulates the lipid metabolism in cancer cells (20).
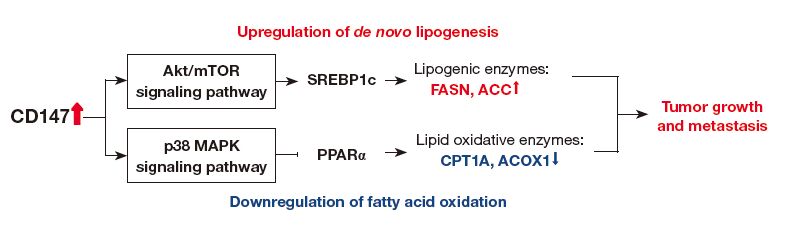
By analyzing four public datasets of mRNA expression in HCC tissues and performing experiments using two different HCC cell lines, Li et al. demonstrated that CD147 significantly contributed to the reprogramming of fatty acid metabolism in HCC cells. They investigated the levels of expression of lipogenic enzymes and sterol regulatory element binding proteins (SREBPs), and activation of Akt/mTOR signaling pathways in tumor cells with different CD147 expression levels. Their data showed that CD147 activated the Akt/mTOR signaling pathway and subsequently up-regulated SREBP1c, leading to the increase in transcription of major lipogenic genes, FASN and ACC1 to promote de novo lipogenesis.
Next, they analyzed the signaling pathway involved in CD147-induced peroxisome proliferator-activated receptor alpha (PPARα) regulation. To test whether CD147 inhibits the expression of PPARα via activation of P38 MAPK signaling pathway, they treated P38 inhibitor SB203580 to CD147-wild type, CD147-knockout and CD147- overexpression HCC cells. They found that the inhibition of P38 MAPK activity up-regulated PPARα in CD147-wild type and CD147-overexpression cells. As well, the treatment with SB203580 led to the activation of FAO and decreases in the contents of triglyceride, phospholipids and neutral lipids. The results suggest that the inhibition of P38 MAPK reversed the down-regulation of FAO by CD147 through the up-regulation of PPARα in HCC cells. Lastly, they found that CD147 knockout or knockdown significantly inhibited the proliferation, migration and invasion of HCC cells, determined via the MTT assay, wound-healing migration assay, trans-well invasion assay, and orthotopic xenograft models. Li et al. verified that CD147 increased the aggressiveness of HCC cells through the Akt/mTOR/ SREBP1c and P38/PPARα pathways, leading to the upregulation of fatty acid synthesis and down-regulation of fatty acid oxidation (Figure 1).
Tumors expressing high levels of CD147 include carcinomas of the urinary bladder, breast, lung, oral cavity, esophagus, skin, and etc. (21-29). It would be interesting to see if CD147 functions likewise as a critical regulator of fatty acid metabolism in other types of cancer. The study by Li et al. is expected to provide new insights into understanding the mechanisms underlying the reprogramming of lipid metabolism in HCC and its association with HCC proliferation and progression, as wells as new strategies for future drug development for HCC treatment.
Acknowledgements
Funding: This work was supported by the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education (NRF-2011-0021830 to SW Ro).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baenke F, Peck B, Miess H, et al. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech, 2013;6 :1353–63. [PubMed]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer, 2007; 7:763–77. [PubMed]
- Glaysher J. Lipid metabolism and cancer. Curr Opin Lipidol, 2013; 24:530–1. [PubMed]
- Currie E, Schulze A, Zechner R, et al. Cellular fatty acid metabolism and cancer. Cell Metab, 2013; 18:153–61. [PubMed]
- Abramson HN. The lipogenesis pathway as a cancer target. J Med Chem, 2011; 54:5615–38. [PubMed]
- Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis, 2010; 31:1509–15. [PubMed]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J, 2012; 279:2610–23. [PubMed]
- Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care, 2006; 9:358–65. [PubMed]
- Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res, 2012; 72:3709–14. [PubMed]
- Rotondo D, Davidson J. Genetics and molecular biology: fatty acid metabolism in cancer cell survival; carnitine palmitoyltransferase-1 as a critical anticancer target. Curr Opin Lipidol, 2011; 22:428–9. [PubMed]
- Huang J, Viswakarma N, Yu S, et al. Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. Am J Pathol, 2011; 179:703–13. [PubMed]
- Xu J, Xu HY, Zhang Q, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res, 2007; 5:605–14. [PubMed]
- Tang X, Guo N, Xu L, et al. CD147/EMMPRIN: an effective therapeutic target for hepatocellular carcinoma. J Drug Target .2012[Epub ahead of print].
- Ma J, Wang JH. 131 I-Labeled-Metuximab plus transarterial chemoembolization in combination therapy for unresectable hepatocellular carcinoma: results from a multicenter phase IV clinical study. Asian Pac J Cancer Prev, 2015; 16:7441–7. [PubMed]
- Bian H, Zheng JS, Nan G, et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radio frequency ablation. J Natl Cancer Inst .2014;106. pii: dju239.
- He Q, Lu WS, Liu Y, et al. 131I-labeled metuximab combined with chemoembolization for unresectable hepatocellular carcinoma. World J Gastroenterol, 2013; 19:9104–10. [PubMed]
- Ke X, Fei F, Chen Y, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis, 2013;33; 1598:607.
- Huang Q, Li J, Xing J, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol, 2014; 61:859–66. [PubMed]
- Huang P, Chang S, Jiang X, et al. RNA interference targeting CD147 inhibits the proliferation, invasiveness, and metastatic activity of thyroid carcinoma cells by down-regulating glycolysis. Int J Clin Exp Pathol, 2015; 8:309–18. [PubMed]
- Li J, Huang Q, Long X, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/ mTOR/SREBP1c and P38/PPARα pathways. J Hepatol, 2015; 63:1378–89. [PubMed]
- Muraoka K, Nabeshima K, Murayama T, et al. Enhanced expression of a tumor-cell-derived collagenase-stimulatory factor in urothelial carcinoma: its usefulness as a tumor marker for bladder cancers. Int J Cancer, 1993; 55:19–26. [PubMed]
- Polette M, Gilles C, Marchand V, et al. Tumor collagenase stimulatory factor (TCSF) expression and localization in human lung and breast cancers. J Histochem Cytochem, 1997; 45:703–9. [PubMed]
- Caudroy S, Polette M, Tournier JM, et al. Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem, 1999; 47:1575–80. [PubMed]
- Bordador LC, Li X, Toole B, et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer, 2000; 85:347–52. [PubMed]
- Ishibashi Y, Matsumoto T, Niwa M, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer, 2004; 101:1994–2000. [PubMed]
- Marionnet C, Lalou C, Mollier K, et al. Differential molecular profiling between skin carcinomas reveals four newly reported genes potentially implicated in squamous cell carcinoma development. Oncogene, 2003; 22:3500–5. [PubMed]
- Thorns C, Feller AC, Merz H. EMMPRIN (CD 147) is expressed in Hodgkin's lymphoma and anaplastic large cell lymphoma. Anticancer Res, 2002; 22:1983–6. [PubMed]
- Nabeshima K, Suzumiya J, Nagano M, et al. Emmprin, a cell surface inducer of matrix metalloproteinases (MMPs), is expressed in T-cell lymphomas. J Pathol, 2004; 202:341–51. [PubMed]
- Nabeshima K, Iwasaki H, Nishio J, et al. Expression of emmprin and matrix metalloproteinases (MMPs) in peripheral nerve sheath tumors: emmprin and membrane-type (MT)1- MMP expressions are associated with malignant potential. Anticancer Res, 2006; 26:1359–67. [PubMed]