Evaluation of rational extent lymphadenectomy for local advanced gastric cancer
Han Liang, Jingyu Deng
Abstract
Based upon studies from randomized clinical trials, the extended (D2) lymph node dissection is now recommended as a standard procedure for local advanced gastric cancer worldwide. However, the rational extent lymphadenectomy for local advanced gastric cancer has remained a topic of debate in the past decades. Due to the limitation of low metastatic rate in para-aortic nodes (PAN) in JCOG9501, the clinical benefit of D2+ para-aortic nodal dissection (PAND) for patients with stage T4 and/or stage N3 disease, which is very common in China and other countries except Japan and Korea, cannot be determined. Furthermore, the role of splenectomy for complete resection of No.10 and No.11 nodes has been controversial, and however, the final results from the randomized trial of JCOG0110 have yet to be completed. Gastric cancer with the No.14 and No.13 lymph node metastasis is defined as M1 stage in the current version of the Japanese classification. We propose that D2+No.14v and +No.13 lymphadenectomy may be an option in a potentially curative gastrectomy for tumors with apparent metastasis to the No.6 nodes or infiltrate to duodenum. The examined lymph node and extranodal metastasis are significantly associated with the survival of gastric cancer patients.
Keywords: Re-evaluation; extended (D2) lymphadenectomy; D2+No.14v lymphadenectomy; para-aortic nodal dissection (PAND)
Submitted Nov 16, 2015. Accepted for publication May 20, 2016.
doi: 10.21147/j.issn.1000-9604.2016.04.02
Introduction
The extent of lymph node dissection during the gastrectomy for resectable advanced gastric cancer (GC) has been debated between the surgeons in East Asia and those in the West. Generally, surgeons in East Asia favored more extensive D2 lymphadenectomy, whereas, surgeons in western countries preferred to use conservative D0 or D1 resection. This is due to the negative results from two earlier randomized trials comparing D1 and D2 resection from the UK and the Netherlands (1, 2). However, this philosophical difference in surgeries for GC has been reduced after the disclosures of another two randomized trials. One of them is from Taiwan, China, in which Wu et al. (3) have demonstrated that modified D3 lymphadenectomy could achieve superior survival compare to D1 resection. The other is from the Dutch trial (4) in which it has shown that after a median follow-up of 15 years, D2 lymphadenectomy is associated with lower locoregional recurrence and GC-related death rates than D1 surgery. According to the Dutch trial, D2 lymphadenectomy (without routine pancreatectomy and splenectomy) is a recommended surgical approach for patients with resectable local advanced GC by all western guidelines, especially in specialized centers with appropriate surgical expertise and postoperative care (5, 6). In East Asia, guidelines from China, Korea and Japan all recommend D2 lymphadenectomy as a standard procedure for locally advanced GC (7-9). Nevertheless, to the disappointment of the investigators, the survival curves of the two arms of JCOG9501 (10) were almost identical, with little room to argue that the para-aortic node dissection (PAND) should still be performed in the prophylactic setting. Other clinical trials conducted in Poland (11), and in the Far East including Japan, Korea and Taiwan, China (12) did not make any meaningful impact on this situation. Therefore, para-aortic nodes (PAN) metastasis secured its place as "distant metastasis" and should be designated as M1 disease.
This review will look into some of the issues that remain to be resolved in the guidelines for GC and try to provide some insights on the optimal extent of dissected lymph nodes (LNs) for different locations and stages of the disease.
The optimal number of dissected LNs for different stage diseases
The 7th edition (TNM classification, the N stages) states that histological examination of a regional lymphadenectomy specimen will ordinarily include 16 or more LNs (13). But the optimal number of LNs to be removed and examined to achieve an optimum reliability in stage assignment remains less clear. It is known that the LN metastasis can occur during the early stages of GC, however, the extent of lymphadenectomy to achieve the optimal result is still not clear and there is no worldwide consensus. Once extragastric LN metastasis is identified, the probability of systemic dissemination of tumor cells will significantly increase. A recent study from the United States (US) Gastric Cancer Collaborative analyzed patients who underwent gastrectomy for gastric adenocarcinoma from 2000 to 2012 at 7 US academic institutions (14). In this report, 742 patients were studied, 257 (35%) had 7 to 15 LNs removed and 485 (65%) had more than 16 LNs removed. The results showed that the 10-year survival rate was 74% and 57% respectively for patients with stage IA-IIIA, or, 72% and 55% respectively for patients with stage N0-N2, both significantly improved (P=0.018 and P=0.023, respectively). Similarly, the German Gastric Cancer Study of 1, 654 patients showed that dissection of >25 LNs had a significant and independent effect on survival in patients with stage II tumors (15).
Study on patients with negative LNs (16) indicated that the survival rate for pT3 tumor patients with at least 25 LNs dissected was significantly higher than the same type of patients but with only 15 to 24 LNs dissected (P=0.019). We previously showed that for patients with low and undifferentiated GC and no metastasis of LNs, 21-30 LNs dissection during the radical gastrectomy may improve the long-term survival (17). We also showed that D2 plus PAND with at least 30 LNs examined may improve the overall survival (OS) for GC patients in N3 stage disease (18). Clinicopathological studies from 769 GC patients who underwent curative gastrectomy with lymphadenectomy between 1997 and 2006 were retrospectively analyzed by Cox regression multivariate analysis, the 7th edition of TNM classification, the number of negative nodes, the type of gastrectomy and the depth of tumor invasion (T stage) were identified as independent factors for predicting the OS of GC patients. We confirmed that the T stage-N stage-Number of negative nodes-Metastasis (TNnM) classification is the most appropriate prognostic predictor of GC patients by case-control matched fashion and multinomial logistic regression (19). With Memorial-Sloan Kettering nomogram (20) created from 1, 039 patients who underwent R0 resection for gastric adenocarcinoma, the number of negative LNs was found to provide prognostic information for disease-specific survival. All these data demonstrate that at least in the subset of GC patients in less advanced stage, a more extensive lymphadenectomy may be associated with prolonged survival.
There are several aspects in our studies that need to be carefully considered. Firstly, patients with more LNs harvested may have improved survival simply because they had a more aggressive procedure, including more extensive LN dissection. Secondly, the number of LNs removed reflects not only the number of LNs dissected during the operation, but also the number of LNs identified while pathologists inspecting the specimen. Lastly, stage migration is a particular challenge for any analysis of this kind. Our previous study indicated that the ratio of positive LNs against the number of LNs examined (RML) was decreased as negative LN (NLN) count increased (P<0.001) (19). Schwarz et al. (21) reported that higher NLN count was correlated with better survival of GC patients after curative resection. This study demonstrated that the best survival results were observed with the NLN count between 15-19 for the T2bN2 patients and 10-14 for the T3N3 patients. When the NLN count increases, the chance of micro-metastasis remaining within NLNs decreases. Standard pathologic examination of LNs is based on hematoxylineosin staining of a single section through hilus. Recent data demonstrate that immunohistochemical evaluation of histological NLNs detects about 30% of micro-metastatic disease (22).
Prognostic significance for patients with/without extranodal metastasis
Extranodal metastasis (EM) is defined as the presence of tumor cells in the extramural soft tissue found during the routine examination of about 10%-20% of resected gastric carcinoma specimens (23, 24). According to the 5th edition of Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM classification (25), this type of tumor spread should be regard as LN metastasis if the nodule has the form and smooth contour of a LN, but should otherwise be regarded as part of the primary tumors. Etoh et al. (26) reported that EM was detected in 146 (14.3%) of the 1, 023 patients, and in 1, 060 (3.0%) of the 35, 811 nodules retrieved as LNs from adipose connective tissues. This study showed that the incidence of EM was significantly higher in patients with tumors that were large, infiltrative, deeply invading or undifferentiated. The same study showed that EM was also significantly higher in patients with LNs, peritoneal or liver metastases, and those with lymphatic or vascular involvement. EM is an independent prognostic factor and should be included in the TNM staging system. A report from Korea (27) assessed the prognostic value of extranodal extension (ENE) in patients with early GC. The 5-year survival rate of patients with ENE was 48.1%, compared with 78.2% for patients without ENE (P<0.001). In patients with early GC, ENE was associated with a worse 5-year survival rate in patients with early (T1) GC (75.0% in patients with ENE and 96.9% in those without ENE, P<0.001).
Our previous study indicated that EM was found in 58 (21.0%) of 276 patients. The 2-, 3-, and 5-year OS rates of the patients with EM were significantly lower than those without EM (71.2%, 55.4% and 45.1%, respectively, vs. 24.1%, 15.5% and 8.0%, respectively, P=0.000) (28). Recently, we evaluated the effect of node-extranodal soft tissue (pNE) stage based on EM, recurrence and survival in patients with GC. All Patients were divided into three groups according to the number of EM (EM0, n=0; EM1, n=1; EM2, n≥2). According to the number of EM, we incorporated EM of GC into pN stage, and consequently introduced the new pNE stage. After comparing with the 7th UICC/AJCC pN stage, we found that the pNE classification [hazard ratio (HR)=1.730, P<0.001] was more appropriate for predicting the OS of GC patients after curative surgery. The -2loglikelihood of the pNE staging (4, 533.991) is smaller than the value of pN (29).
Therefore, EM is closely associated with cancer aggressiveness and the presence of EM is a significant independent predictor of reduced disease-free survival (DFS) and OS in patients with early or locally advanced GC. EM is an independent risk factor for distance recurrence, especially for peritoneal recurrence, and the selection of postoperative systemic (intravenous or intraarterial) or regional (intraperitoneal) therapy basing on the status of EM may be a reasonable approach. As an important prognostic factor, EM should be incorporated into N stage according to its number retrieved in postoperative samples.
Splenectomy or spleen-preservation for proximal GC which does not invade great curvature
The frequency of No.10 node metastasis was reported to be high in proximal advanced GC located on the greater curvature or in the posterior wall of stomach. Also, lymphatic pathways along the posterior gastric artery, splenic artery, short gastric vessels, and/or gastroepiploic vessels were suggested to be important for No.10 node metastasis (30). A report from Japan indicated that the location involving the great curvature, pN3 and No.11d metastasis were risk factors for No.10 node metastasis and the frequency of No.10 node metastasis was similar to that of No.4sb, No.9, and No.11p metastasis. Furthermore, LN dissection effect index of No.10 was almost as same as that of No.9, No.11p, and No.11d (31). A report from China demonstrated that the survival rates of splenectomy group and spleen-preserving group were 30.0% and 19.7%, respectively, whose difference was significant (P<0.05), indicating the splenectomy was an independent prognostic factor. Therefore, total gastrectomy with splenectomy is recommended for patients with No.10 node positive T3 proximal GC (32). A meta-analysis of 2, 628 patients from 12 studies comparing outcomes after radical resection of GC with or without splenectomy indicates that radical resection of GC combined with splenectomy is not associated with improved survival but has instead increased postoperative complications (33). Accordingly, the splenectomy for D2 lymphadenectomy may be unnecessary in all the patients with advanced disease since patients with No.10 node metastasis had already have too extended LN metastasis to improve the prognosis. Recently some Japanese surgeons introduced a policy of splenectomy to the patients with No.10 node enlargement in splenic hilum, and in those cases, the metastasis or tumor location is possibly in greater curvature or encircling in the upper third of stomach (31).
A randomized controlled trial, which recruited 505 patients, has been carried out to evaluate total gastrectomy with splenectomy for proximal advanced GC with R0 resection (JCOG0110-MF) (34). The final long-term survival of this trail has been reported by Prof. Sano at the 11th International Gastric Cancer Congress in Sao Paulo in June 04, 2015. Over a topic section in the conference, surgical approaches toward splenic hilum nodes and distal splenic artery nodes have been discussed. According to the results of JCOG0110-MF, it is suggested that in total gastrectomy for proximal GC with no great curvature invasion, prophaylactic splenectomy should be avoided not only for operative safety but also for the survival benefit. According to the opinion from Prof. Sano, spleen should be preserved even in case the great curvature is involved, unless the gastrosplenic ligament is directly invaded. No.10 LNs should be dissected without splenectomy.
No.14v node dissection for distal local advanced GC
According to the Japanese Gastric Cancer Treatment Guidelines 2010 (ver.3) (9), the role of No.14v lymphadenectomy in distal GC is controversial. Dissection of node No.14v as part of D2 gastrectomy defined in the second edition of the Japanese classification has been excluded from the current edition. However, D2+No.14v may be beneficial in tumors with apparent metastasis to the No.6 nodes. A retrospective study from Korea (35) demonstrated that the No.14v status is an independent prognostic factor with stage IV cancer, with 14v-positive GC having a poor prognosis, similar to that of M1 disease. Therefore, the exclusion of No.14v in regional LN dissection should be considered. However, a subgroup analysis is lacking there and the metastatic rate for No.14v is only 6%. Our studies on patients with middle and distal advanced GC showed that the metastatic rate for No.14v nodes was 18.3%-19.4% (36, 37).
Our recent published data including 243 patients with No.14v LN dissection, among which 45 patients (18.5%) had No.14v metastasis (38). Only one patients with stage I and II had No.14v metastasis. The frequency of No.14v involvement was 6.3%, 20.5% and 32.2% respectively in stage IIIa, IIIb and IIIc and it rose to 66.7% in stage IV disease (Table 1). As patients with No.14v metastasis fall mainly in stage TNM III/IV, we have stratified patients with TNM staging. The OS difference between No.14v positive patients and negative ones was only observed in stage III GC patients, and the OS rate for patients with No.14v metastasis was significantly lower (5-year OS: 42.9% vs. 57.6%, P=0.005). No.14v node metastasis was found to correlate significantly with the tumor location (region including the lower third of the stomach), the depth of invasion [muscularis propria (MP) or deeper] and N stage. The odds ratio of No.6, No.8a was high (16.83 and 8.37) in compare with other LNs. D2 lymphadenectomy in combination with No.14v LN seemed to have improved the OS for clinical stage III/IV GC located in the middle or lower third of stomach.
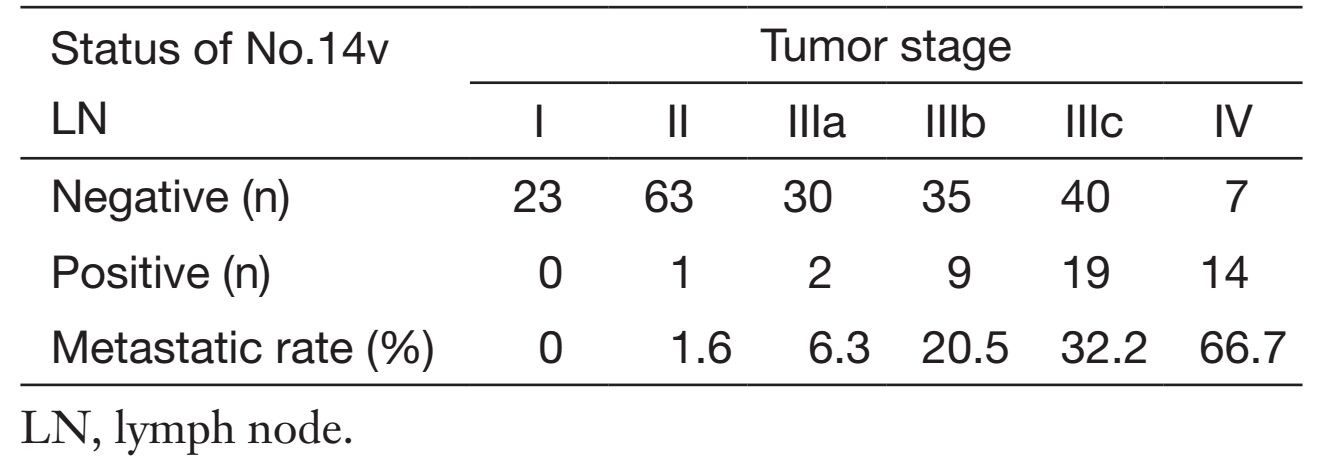
Full table
There are several conceivable reasons for this: firstly, if undetectable microscopic metastasis, 29.5% in some studies (22), is present in No.14v, systemic dissection of that area could be helpful to avoid metastasis to adjacent retroperitoneal LNs. Secondly, No.14v lymphadenectomy might make the No.6 LN dissection more complete in cases with No.6 LN metastasis. The area of No.6 LN can be somewhat ambiguous in some occasions due to some common anatomic variations of the venous structure. Therefore, the expansion of the LN dissection area to the No.14v could improve the curability when metastasis happens in the infrapyloric area. Recently we conducted a multi-center prospective randomized clinical trial (NCT02272894) including more than 20 Chinese institutions on D2 plus lymphadenectomy along the superior mesenteric vein in locally advanced (T3-4N+) middle and lower GC to elucidate the potential impact of No.14v node dissection on long-term survival of GC cancer patients.
Sasako et al. (39) reported a new method in 1995 to evaluate the therapeutic value of LN dissection. In this method, therapeutic value of extended LN dissection was estimated by multiplying the number of metastasized LN by 5-year survival rate of patient with metastasis at each station. The index of estimated benefit of No.14v was 2.1, which was similar to that of No.1 (1.6) and No.12a (2.7). A later report from Japan indicated that the therapeutic index for dissection of No.14v was 5.39, again, similar to that of No.5 (5.93) (40).
PAND and its prognostic benefit for patients with locally advanced GC
Gastrectomy with D2 lymphadenectomy is the standard treatment for curable GC in East Asia, the improved survival rate for additional PAND to D2 lymphadenectomy for locally advanced disease is controversial. According to the current TNM classification, PAN involvement is considered a distant metastasis (M1), and the incidence of metastasis to PAN is present in 18%-40% of advanced GCs. In our study, the incidence of PAN metastasis was 27.0% (47/174) (18), which is similar to the findings reported in some other studies (41, 42). Natsugoe et al. (43) reported that the incidence of micro-metastasis in PAN was up to 64%.
The result of the JCOG9501 trial demonstrated that the 5-year OS rate was 69.2% for the group assigned to D2 lymphadenectomy alone and 70.3% for the group assigned to D2+PAND (10). The criteria of eligibility for the JCOG9501 trial were patients with stage T2b, T3 or T4. But when we look into the final enrolled patients in both groups, we can find that there were almost only patients with T2-3, N1-2 (IIB/IIIA) disease, and only 1.9% for patients with T4 underwent D2+PAND and 3.0% for patients with T4 underwent D2 procedure. Therefore, the objective conclusion for the JCOG9501 trial is that patients with T2b-3, N1-2 (IIB-IIIA) should not be treated with D2+PAND but there is no decent indications on the patients with T4N3 (IIIB/IIIC) disease. Evidence from later studies suggested a strong possibility that the D2+PAND can benefit selected patients with GC (44, 45).
We retrospectively studied 174 GC patients who underwent gastrectomy with D2+PAND from January 2001 to December 2010 (46). The total number of LNs dissected was 5, 568 from all patients and the median number was 29.5 (range: 15-72) nodes per patient. Within the dissected nodes, a total of 1, 287 LNs were identified as positive from 124 (71.3%) patients. The median number of metastatic nodes was 7 (range: 1-60). In addition, a total of 594 LNs were harvested from station 16, with a median number of 2 (range: 1-15) nodes per patient. In those LNs, PAN metastasis was identified in a total number of 149 LNs in 47 patients (27.0%), and the median number was 2 (range: 1-14) per patient. According to the analysis of clinicopathologic characteristics, we concluded: 1) tumors from the lower third of stomach had a lower likelihood of PAN metastasis. Metastases to PAN were found in 17.9% (14/78) of the cases when tumor was located in the lower third, but metastases to PAN were found in more than 30% of cases when cancer arose from the upper-middle third or occupied more than one-third; 2) the incidence was significantly higher in patients when the primary tumor type was Borrmann III (31/99, 31.3%) or IV (8/17, 47.1%), whereas the incidence was 13.8% (8/58) for Borrmann type I or II; and 3) higher pathologic N stage is coupled with greater incidence of PAN metastases. The incidence of PAN metastasis increases from 16.1% (5/31) in N1 cases and 23.3% (7/30) in N2 cases to 41.2% (14/34) in N3a cases and 69.0% (20/29) in N3b cases. N stage was a significant risk factor for PAN metastasis after adjusting for other variables.
All these data demonstrate that N stage and perigastric nodal status are important and independent risk factors for PAN metastasis, which can be used for identifying patients with high risk of PAN metastasis who can benefit from PAN dissection (47). Another study from our group indicated that 34.1% of patients with stage N3 disease had PAN metastasis. Therefore, patients with stage N3 disease should at least have 30 LNs examined, with D2 LN dissection plus PAND to improve the OS (18).
As JCOG9501 shows, D2+PAND can be performed by specialized surgeons without increasing major surgical complications and LN dissection does not adversely influence quality of life (10). D2 surgery plus PAND can be performed for patients with a high incidence of PAN metastasis. Prof. Sasako made a keynote speech about this at the 11th International Gastric Cancer Congress in Sao Paulo in June 4, 2015. He indicated that, according to the JCOG0001 (48) and JCOG0405 (49, 50), for patients with clinically detected PAN metastasis or bulky nodal metastasis surrounding the branches of celiac artery without clinical PAN metastasis, PAND should be carried out after intensive chemotherapy. In term of the PAND application when performing R0 resection, Prof. Sasako stated that the definite indication for performance is clinical PAN metastasis limited to No.12a2 and No.16b1 or bulky N2 with or without PAN metastasis. Prof. Sasako also stated that the potential indication for PAND performance is esophagogastric junction adenocarcinoma Siewert Type II or III.
Based on our experience and literature researches, the following are indications for D2+PAND candidates: 1) patients in good condition with no serious organ dysfunction; 2) patients without peritoneal dissemination or liver metastases; 3) patients with pathologic N2, N3a and N3b stage disease or positive No.9 LN; 4) patients with Borrmann type III/IV disease; and 5) patients with upper-middle third or occupied more than one-third. However, we recommend that D2+PAND should be carried out only in cancer centers equipped with surgeons with extensive experience for extended LN dissections, because there are some risks in some rare situations, such as complications like formation of chylous fistula. In addition, multiple methods should be used in selecting the suitable cases for further study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomized controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995–9. [PubMed]
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745–8. [PubMed] DOI:10.1016/S0140-6736(95)90637-1
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309–15. [PubMed] DOI:10.1016/S1470-2045(06)70623-4
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49. [PubMed] DOI:10.1016/S1470-2045(10)70070-X
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013(11): 531–46. [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Radiother Oncol 2014;110:189–94. [PubMed] DOI:10.1016/j.radonc.2013.09.015
- Ministry of Health of the People’s Republic of China. Gastric cancer treatment guideline 2011. Available online: http://www.nhfpc.gov.cn/yzygj/s3585u/201103/96ce9016858d416f83da688df45e3f2e.shtml
- Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87–104. [PubMed] DOI:10.5230/jgc.2014.14.2.87
- Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23.
- Sasako M, Sano T, Yamamoto S, et al. D2 lymph-adenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453–62. [PubMed] DOI:10.1056/NEJMoa0707035
- Kulig J, Popiela T, Kolodziejczyk P, et al. Standard D2 versus extended (D2+) lymphadenectomy for gastric cancer: an interim safety analysis of a multicenter, randomized, clinical trial. Am J Surg 2007;193:10–5. [PubMed] DOI:10.1016/j.amjsurg.2006.04.018
- Yonemura Y, Wu CC, Fukushima N, et al. Randomized clinical trial of D2 and extended paraaortic lymph-adenectomy in patients with gastric cancer. Int J Clin Oncol 2008;13:132–7. [PubMed] DOI:10.1007/s10147-007-0727-1
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Oxford: Wiley-Blackwell; 2009 .
- Gholami S, Janson L, Worhunsky DJ, et al. Number of lymph nodes removed and survival after gastric cancer resection: an analysis from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:291–9. [PubMed] DOI:10.1016/j.jamcollsurg.2015.04.024
- Siewert JR, B?ttcher K, Stein HJ, et al. Relevant prognostic factor in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449–61. [PubMed] DOI:10.1097/00000658-199810000-00002
- Jiao XG, Deng JY, Zhang RP, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol 2014;20:3640–8. [PubMed] DOI:10.3748/wjg.v20.i13.3640
- Jiang N, Deng JY, Liu Y, et al. Prognostic factor of low- and undifferentiated gastric cancer with negative metastasis of lymph nodes. Zhongguo Xiao Hua Wai Ke Za Zhi (in Chinese) 2014;13:629–32.
- Liang YX, Liang H, Ding XW, et al. The prognostic influence of D2 lymphadenectomy with para-aortic lymph nodal dissection for gastric cancer in N3 stage. Zhonghua Wai Ke Za Zhi (in Chinese) 2013;51:1071–6.
- Deng J, Liang H, Wang D, et al. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol 2010;17:1043–51. [PubMed] DOI:10.1245/s10434-009-0863-0
- Kattan MW, Karpeh MS, Mazumdar M, et al. Post-operative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol 2003;21:3647–50. [PubMed] DOI:10.1200/JCO.2003.01.240
- Schwarz RE, Smith DD. Clinical impact of lymphadenec-tomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317–28. [PubMed] DOI:10.1245/s10434-006-9218-2
- Xu KF, Zhou YB, Li Y, et al. Study on metastasis and micrometastasis in No.14v lymph nodes of patients with lower third gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2011;14:125–7.
- Tanaka T, Kumagai K, Shimizu K, et al. Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J Surg Oncol 2000;75:165–71. [PubMed] DOI:10.1002/(ISSN)1096-9098
- Itoh H, Hase K, Suganuma T, et al. Clinical value of extramural and extranodal cancer permeation as a prognostic indicator in patients with gastric cancer. Jpn J Gastroenterol Surg 2002;35:1475–81. DOI:10.5833/jjgs.35.1475
- Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803–4. [PubMed] DOI:10.1002/(ISSN)1097-0142
- Etoh T, Sasako M, Ishikawa K, et al. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg 2006;93:369–73. [PubMed] DOI:10.1002/(ISSN)1365-2168
- Lee IS, Park YS, Ryu MH, et al. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg 2014;101:1576–84. [PubMed] DOI:10.1002/bjs.2014.101.issue-12
- Wang XN, Ding XW, Zhang L, et al. Correlation analysis of gastric cancer with extranodal metastasis. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2007;10:436–9.
- Jiang N, Deng JY, Ding XW, et al. Node-extranodal soft tissue stage based on extranodal metastasis is associated with poor prognosis of patients with gastric cancer. J Surg Res 2014;192:90–7. [PubMed] DOI:10.1016/j.jss.2014.05.053
- Aoyagi K, Kouhuji K, Miyagi M, et al. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol 2010;2:81–6. [PubMed]
- Nashimoto A, Yabusaki H, Matsuki A. The significance of splenectomy for advanced proximal gastric cancer. Int J Surg Oncol 2012;2012:301530. [PubMed]
- Huang CM, Wang JB, Lu HS, et al. Prognostic impact of splenectomy on advanced proximal gastric cancer with No. 10 lymph node metastasis. Chin Med J (Engl) 2009;122:2757–62. [PubMed]
- Ding J, Liao GQ, Zhang ZM, et al. Necessity of splenectomy in radical resection of gastric cancer: a meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2001;14:120–4.
- Sano T, Yamamoto S, Sasako M, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 2002;32:363–4. [PubMed] DOI:10.1093/jjco/hyf085
- An JY, Pak KH, Inaba K, et al. Relevance of lymph node metastasis along the superior mesenteric vein in gastric cancer. Br J Surg 2011;98:667–72. [PubMed] DOI:10.1002/bjs.v98.5
- Liang YX, Liang H, Ding XW, et al. Significance of No.14v lymph node dissection for advanced gastric cancer undergoing D2 lymphadenectomy. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2013;16:632–6.
- Jiao X, Liang H, Deng J, et al. Risk factors for group 14v lymph node metastasis in advanced gastric cancer. Zhonghua Xiao Hua Wai Ke Za Zhi (in Chinese) 2014;13:3.–3.
- Liang Y, Wu L, Wang X, et al. Positive impact of adding No.14v lymph node to D2 dissection on survival for distal gastric cancer patients after surgery with curative intent. Chin J Cancer Res 2015;27:580–7. [PubMed]
- Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346–51. [PubMed] DOI:10.1002/(ISSN)1365-2168
- Tokunaga M, Ohyama S, Hiki N, et al. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial. Ann Surg Oncol 2009;16:1241–6. [PubMed] DOI:10.1245/s10434-009-0345-4
- Kunisaki C, Shimada H, Yamaoka H, et al. Significance of para-arotic lymph node dissection in advanced gastric cancer. Hepatogastroenterology 1999;46:2635–42. [PubMed]
- Hsu CP, Wu CC, Chen CY, et al. Clinical experience in radical lymphadenectomy for adenocarcinoma of the gastric cardia. J Thorac Cardiovasc Surg 1997;114:544–51. [PubMed] DOI:10.1016/S0022-5223(97)70042-4
- Natsugoe S, Nakashima S, Matsumoto M, et al. Paraaortic lymph node micrometastasis and tumor cell microinvolvement in advanced gastric carcinoma. Gastric Cancer 1999;2:179–85. [PubMed] DOI:10.1007/s101200050043
- Zhang H, Liu C, Wu D, et al. Does D3 surgery offer a better survival outcome compared to D1 surgery for gastric cancer? A result based on a hospital population of two decades as taking D2 surgery for reference. BMC Cancer 2010;10:308. [PubMed] DOI:10.1186/1471-2407-10-308
- Zhang C, He Y, Schwarz RE, et al. Evaluation of para-aortic nodal dissection for locoregionally advanced gastric cancer with 1-3 involved para-aortic nodes. Chin Med J (Engl) 2014;127:435–41. [PubMed]
- Wang L, Liang H, Wang X, et al. Risk factors for metastasis to para-aortic lymph nodes in gastric cancer: a single institution study in China. J Surg Res 2013;179:54–9. [PubMed] DOI:10.1016/j.jss.2012.08.037
- McCulloch P, Niita ME, Kazi H, et al. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg 2005;92:5–13. [PubMed] DOI:10.1002/bjs.v92:1
- Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015–22. [PubMed] DOI:10.1002/bjs.v96:9
- Yoshikawa T, Nakamura K, Tsuburaya A, et al. A phase II study of preoperative chemotherapy with S-1 (S) and cisplatin (P) followed by D3 gastrectomy for gastric cancer (GC) with extensive lymph node metastasis (ELM): Survival results of JCOG0405. J Clin Oncol 2011;29(4 supp1):abstr 70.
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653–60. [PubMed] DOI:10.1002/bjs.9484
