A phase I trial of an oral subtype-selective histone deacetylase inhibitor, chidamide, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer
Xingsheng Hu1*, Lin Wang1*, Lin Lin1, Xiaohong Han1, Guifang Dou2, Zhiyun Meng2, Yuankai Shi1
*These authors contributed equally to this work.
Abstract
Objective: This phase I study was to evaluate safety, maximum tolerated dose, pharmacokinetics and preliminary antitumor activity of chidamide, a novel subtype-selective histone deacetylase (HDAC) inhibitor, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer (NSCLC).
Methods: Ten patients received oral chidamide 20, 25, or 30 mg twice per week continuously with paclitaxel (175 mg/m2) and carboplatin [area under the curve (AUC) 5 mg/mL/min] administered in a 3-week cycle. Patients with response and stable disease after four cycles maintained chidamide monotherapy until disease progression or unacceptable toxicity. Blood samples were collected for pharmacokinetic analysis after the first single oral of chidamide and first combination treatment in cycle 1 from all patients.
Results: Two dose-limiting toxicities were recorded in the 30 mg cohort, including thrombocytopenia and prolonged neutropenia in the first cycle. Grade 3/4 neutropenia in any cycle was observed in all patients, but was not associated with significant complications. Other grade 3/4 hematologic toxicities included thrombocytopenia and leucopenia. No significant changes were observed in pharmacokinetic parameters for both chidamide and paclitaxel. One patient in the 20 mg cohort had confirmed partial response (PR). Two out of 5 patients with brain metastases had intracranial complete remission after 4-cycle treatment.
Conclusions: Chidamide combined with paclitaxel and carboplatin was generally tolerated without unanticipated toxicities or clinically relevant pharmacokinetic interactions. The recommended dose for chidamide in this combination was established at 20 mg, and a phase II trial is ongoing with this regimen in patients with advanced NSCLC.
Keywords: Chidamide; HDAC inhibitor; phase I; paclitaxel and carboplatin; non-small cell lung cancer
Submitted Mar 09, 2016. Accepted for publication Jul 31, 2016.
doi: 10.21147/j.issn.1000-9604.2016.04.08
Introduction
Non-small cell lung cancer (NSCLC) is the most common diagnosed cancer and the leading cause of cancer death worldwide (1, 2). In China, new NSCLC cases are approximately 569, 000 annually (3). Platinum-based chemotherapy remains the first-line treatment for advanced NSCLC, especially in patients without mutations in certain genes such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). However, the efficacy of different platinum-based regimens appears to have reached a plateau, with the median survival ranges from 8 to 12 months (4, 5), even with the addition of targeted agents, such as bevacizumab and cetuximab (6, 7). Novel systemic therapy strategies are in great need to improve the survival in advanced NSCLC.
Chidamide (CS055) is a novel member of the benzamide class of histone deacetylase (HDAC) inhibitor. It was rationally designed to block the catalytic pocket of Class I HDACs with enhanced metabolic stability, durable induction of histone acetylation, and distinct gene expression profiling relative to existing benzamide and hydroxamic acid class inhibitors (8-14). Previous studies have demonstrated that chidamide, functioning as an epigenetic modulator, selectively inhibits the activity of HDAC1, 2, 3 and 10, and performs its antitumor action via multiple mechanisms, including induction of growth arrest and apoptosis in blood and lymphoid-derived tumor cells (9), activation of natural killer cell- and antigen-specific CD8+ cytotoxic T lymphocyte-mediated cellular antitumor immunity (10, 11), and reversion of epithelial-mesenchymal transitions and drug-resistance of tumor cells (12). Chidamide has also been shown to synergistically enhance platinum-induced DNA damage responses and apoptosis in NSCLC cells (13).
Chidamide has shown good tolerability, antitumor activity, and favorable pharmacokinetics and pharmaco-dynamics profiles in phase I and phase II studies in patients with advanced solid tumors or lymphomas (8, 14). Based on the results from a multicenter pivotal phase II study (14), chidamide has been approved for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL) in China. On the basis of the preclinical and clinical findings, we designed this phase I study to evaluate the safety and pharmacokinetics of chidamide in combination with paclitaxel and carboplatin as first-line treatment in patients with advanced NSCLC.
Patients and methods
Patients
Patients (age ≥18 years) with cytologically or histologically confirmed NSCLC, stage IIIB or IV disease, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, treatment-naive or recurrent after surgery without systematic therapy were eligible. Presence of measurable diseases, life expectancy of ≥3 months and body weight 47-87 kg for men and 35-75 kg for women were required. Qualifying laboratory criteria were as follows: leukocytes ≥4×109/L, platelets ≥100×109/L, hemoglobin ≥11 g/dL, total bilirubin ≤1.5× upper limits of normal (ULN), alanine transaminase (ALT)/aspartate aminotransferase (AST) ≤1.5×ULN, and creatinine ≤1.5×ULN.
Pregnant women and men with fertility desires were excluded, and women with reproductive potential were required to use contraception. Other exclusion criteria were: 1) history of QTc prolongation, ventricular tachycardia (VT), auricular fibrillation (AF), heart block (HB), myocardial infarction (MI) onset within one year, congestive heart failure (CHF), or clinically significant coronary artery disease which needs drug treatment; 2) patients who had undergone organ transplantation; 3) patients with active bleeding or newly diagnosed thromboembolic diseases; 4) patients with active ulcer or bleeding in gastrointestinal tract; 5) patients with active infections; 6) patients with symptomatic brain metastases; or 7) patients with mental disorders. EGFR mutation status was not examined in this study.
The study was approved by China Food and Drug Administration (CFDA) and the Ethics Committee of Cancer Institute & Hospital, Chinese Academy of Medical Science & Peking Union Medical College. All patients provided written informed consent before enrollment. The study was registered on World Health Organization International Clinical Trials Registry Platform (registration No. ChiCTR-ONC-12002283), and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Study design
The primary purpose of this open-label study was to determine the recommended phase II dose of chidamide combined with fixed dose of paclitaxel and carboplatin in a 3-week cycle in patients with advanced NSCLC. Chidamide (5 mg tablets) was provided by Chipscreen Biosciences Ltd. (Shenzhen, China). Paclitaxel and carboplatin were commercially available from Bristol-Myers Squibb (New York, USA).
The starting dose of chidamide was 20 mg and escalated by 5 mg with a 3+3 (3 to 6 patients per cohort) "up and down" design. A dose-limiting toxicity (DLT) was defined as the occurrence of any of the following events in treatment cycle 1: 1) grade 4 neutropenia lasting >7 d; 2) grade 3 febrile neutropenia with fever >38.5 ℃; 3) grade 4 thrombocytopenia; 4) grade 3 nausea or vomiting lasting >48 h despite maximal symptomatic treatment; 5) grade 4 nausea or vomiting; 6) grade 3 or higher non-hematologic toxicity excluding nausea, vomiting, or alopecia; 7) cycle 2 treatment delay >2 weeks due to intolerable events; and 8) drug-related and clinically significant grade 3 or higher non-hematologic laboratory abnormity. The grade of toxicities and the relationship of adverse events (AE) with the study drug/combination treatment regimen were evaluated and determined by investigators.
Drug administration and dose modifications
Chidamide tablets were administered orally 30 min after breakfast twice weekly (BIW) on d 1 and d 5 every 3 weeks of each cycle. On d 5 of each cycle, along with oral chidamide, paclitaxel (175 mg/m2) was infused intravenously over 3 h, immediately followed by a 30 min infusion of carboplatin [area under the curve (AUC)=5 mg/mL/min]. Patients with stable disease (SD) or better after four cycles of combination treatment were administered with single chidamide with the same dose as maintenance, until disease progression or unacceptable toxicity. Paclitaxel-treated patients received dexamethasone (20 mg oral doses 12 and 6 h before paclitaxel), diphenhydramine [50 mg intravenous injection (i.v.)], and a histamine receptor 2 antagonist (50 mg i.v. ranitidine or 300 mg i.v. cimetidine).
Dose reductions were required in patients who had no DLT in cycle 1 and experienced grade 3 or higher non-hematologic toxicity, or grade 4 hematologic toxicity, or grade 3 neutropenia lasting >7 d or febrile neutropenia with fever >38.5℃ in the subsequent period. The principle of dose reduction was as follows: 1) if toxicity was chidamide-related, the dose of chidamide was reduced to the next lower level for the subsequent treatment cycles; 2) if toxicity was paclitaxel-related, the dose of paclitaxel was reduced by 25 mg/m2, and if carboplatin-related, the dose of carboplatin was reduced by 1 mg/mL/min; and 3) patients with 2 or more dose reductions should be withdrew from the study.
Pharmacokinetic sampling and analysis
Blood samples (3 mL) for chidamide when given alone (d 1, cycle 1), and with paclitaxel and carboplatin (d 5, cycle 1), were collected from all patients before and 1, 2, 3, 6, 12, 24, 48 and 72 h after administration of chidamide, and after the onset of paclitaxel and carboplatin infusion, respectively.
Plasma concentrations of chidamide and paclitaxel were determined by a validated, sensitive, and specific high-performance liquid chromatography method with tandem mass spectrometric detection (LC-MS/MS) (15). The assay was linear over a range of 1-1, 000 ng/mL with a lower limit of quantification (LLOQ) of 1 ng/mL for chidamide, and 20-10, 000 ng/mL with a LLOQ of 20 ng/mL for paclitaxel, respectively.
Patient evaluation
Baseline evaluations included physical examination, performance status, complete blood count (CBC), biochemistry, urinalysis, electrocardiograph (ECG), cardiac ultrasound and computed tomography (CT) scan. CBC tests were performed BIW in combination treatment period, and once weekly in single chidamide treatment period. ECG examination was performed weekly. Physical examination and laboratory biochemistry were carried out every 3 weeks. Urinalysis and cardiac ultrasound were examined every 6 weeks.
Responses were assessed every 6 weeks by CT scan using Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0), and toxicity was graded using National Cancer Institute the Common Toxicity Criteria (NCI-CTC, version 3.0) for AEs.
Statistical analysis
Median of patient age was calculated based on the baseline data from 10 patients enrolled. The safety population included all patients who received at least one dose of chidamide. All descriptive statistical analyses were carried out by using the SAS software (Version 9.2, SAS Institute Inc., Cary, USA).
Pharmacokinetic parameters of chidamide and paclitaxel were analyzed using non-compartmental methods by Phoenix 6.0 WinNonlin, Version 5.2 (Pharsight Corporation, Mountain View, CA, USA). The maximum plasma drug concentration (Cmax) and time to reach maximum concentration (Tmax) were obtained from experimental observation. The AUC was estimated using a trapezoidal rule. Summary statistics were expressed as x±s.
The paired two-tailed t test was employed using IBM SPSS Statistics (Version 19.0, IBM Corp., New York, USA) to assess mean pharmacokinetic variables for chidamide (before and after combination with paclitaxel/carboplatin) and paclitaxel (among each chidamide combination dose group) in cycle 1 for all the patients, and P<0.05 (two-tailed) was considered statistically significant.
Results
Demographic and baseline patient profile
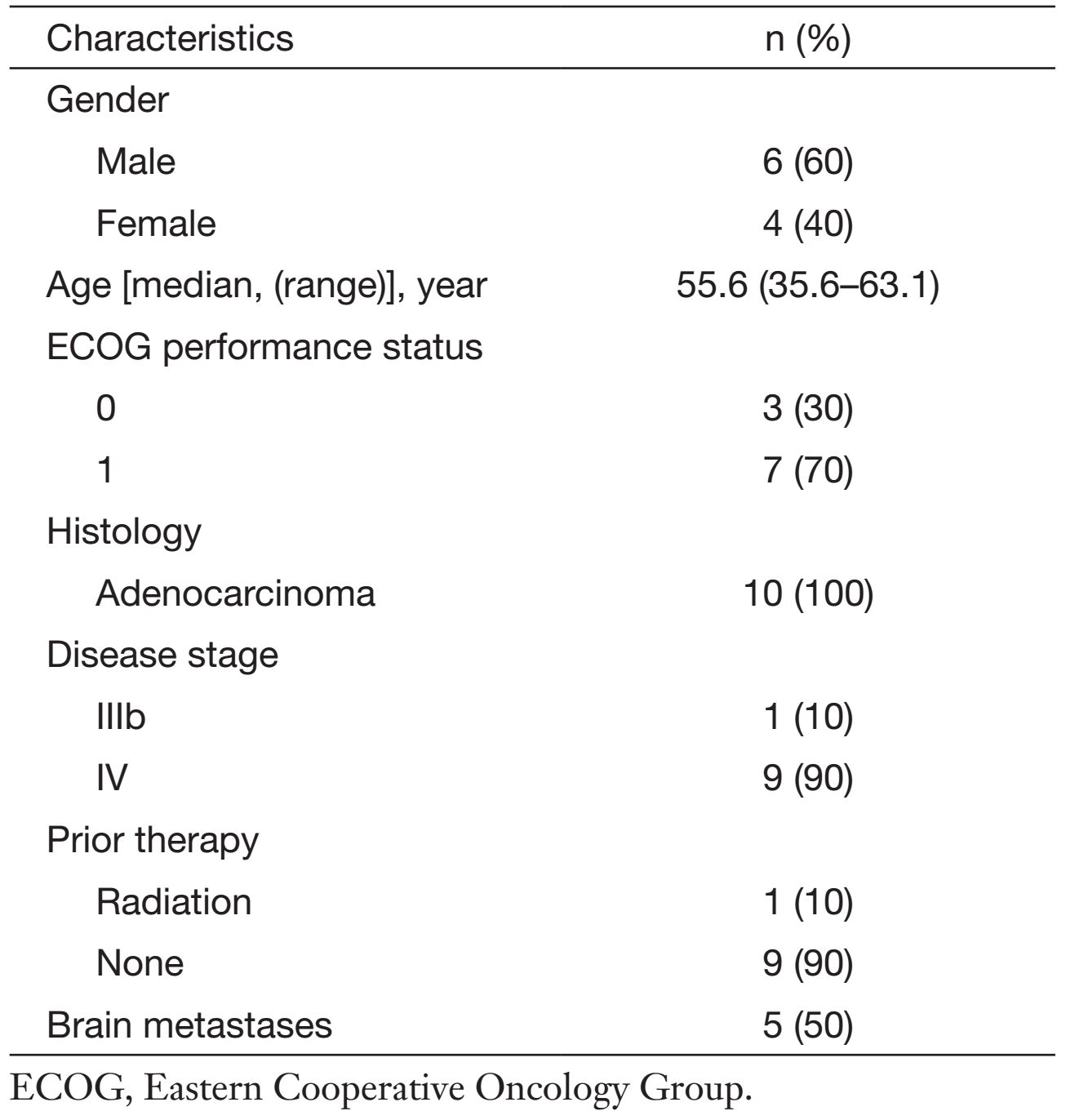
This phase I study was conducted at Cancer Institute & Hospital, Chinese Academy of Medical Sciences. A total of 10 patients were enrolled from March 2011 to April 2012 to receive protocol treatment. As listed in Table 1, the median age was 55.6 (range 35.6-63.1) years old, with 7 patients having an ECOG performance status equal to 1, 3 patients with ECOG performance status equal to 0. All patients had adenocarcinoma with one or more disease sites. Most patients (90%) had stage IV disease. Five patients (50%) had brain metastasis without clinical symptoms. Nine patients (90%) were treatment-naive, and 1 patient received radiotherapy for brain metastasis.
Full table
Patient disposition
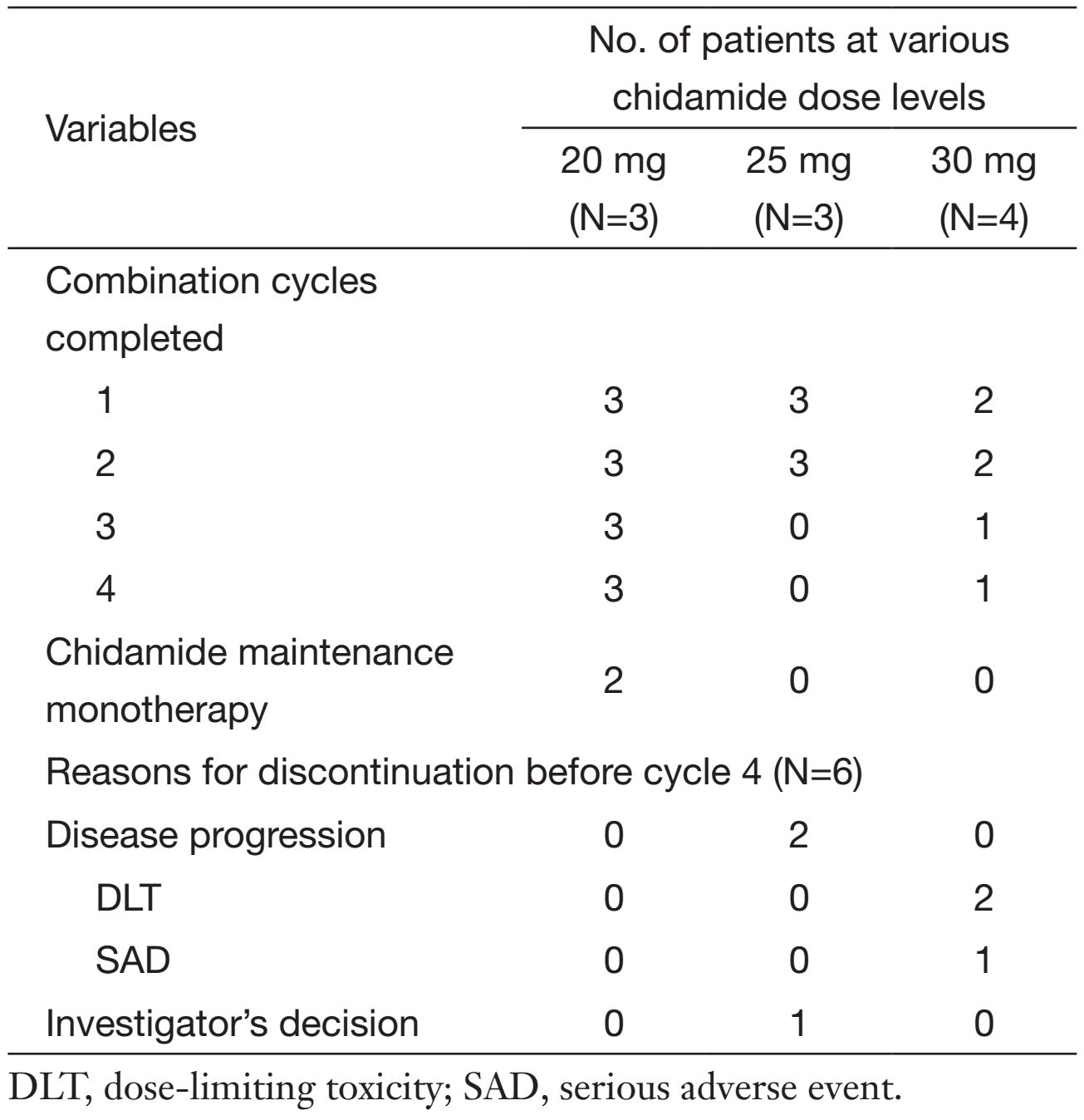
All 10 patients received at least one cycle of combination therapy and were evaluable for DLT and safety. As shown in Table 2, 4 patients completed the 4 scheduled cycles of combination therapy, and 2 patients went on to receive chidamide maintenance monotherapy. Three patients were enrolled into the first dose level (20 mg cohort). No patients in this cohort experienced DLT and all completed the planned 4-cycle combination therapy, in which 2 patients continued to receive the maintenance chidamide. In the 25 mg cohort, 3 patients were enrolled and all treated for 2 cycles without dose reduction. Two patients discontinued treatment due to disease progression, and one due to lack of treatment-benefit based on investigator's discretion. Four patients were enrolled in the 30 mg cohort. One patient completed the 4-cycle combination therapy, 2 patients discontinued treatment in cycle 1 due to DLTs, and 1 patient experienced serious adverse event (SAE) and withdrew from the study.
Full table
Safety evaluation
No DLT was observed in the 20 mg and 25 mg cohorts. Two out of 4 patients in the 30 mg cohort experienced DLTs defined by the protocol: 1 patient had grade 4 thrombocytopenia, and the other had treatment delay over 14 d for the subsequent treatment because of grade 3 neutropenia and grade 2 thrombocytopenia.
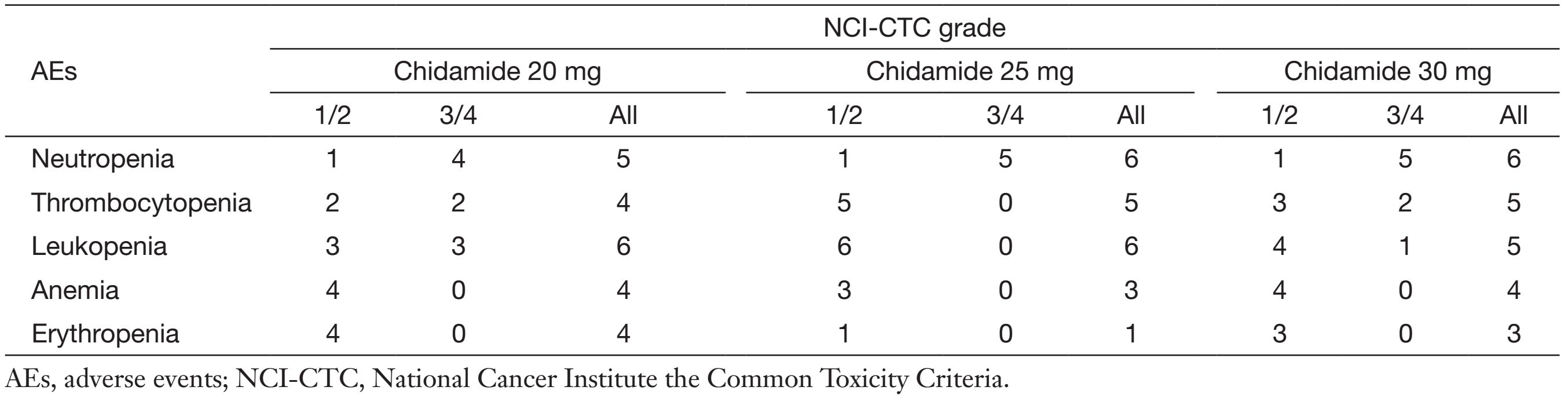
All patients experienced at least one AE. Myelo-suppression, particularly neutropenia, was the principal hematologic toxicity of chidamide/pactlitaxel/carboplatin regimen (Table 3). Grade 3/4 neutropenia was observed in all patients, but not associated with significant complications. Other grade 3/4 hematologic toxicities included thrombocytopenia and leucopenia. Higher severity of hematologic toxicities was recorded in the 30 mg group. Non-hematological toxicities were mild to moderate. Nausea, alopecia and fatigue were relatively common (>50%) non-hematologic toxicities, but all were grade 1. Other non-hematologic toxicities were most grade 1 or grade 2, and only 1 case of grade 3 hypocalcemia was documented in the 20 mg cohort.
Full table
None of the patients had chidamide dose reduction during the study. One patient at the 20 mg dose level and 2 patients at the 30 mg dose level had dose reduction of paclitaxel or carboplatin per the study protocol. There was no treatment-related death in the study. One patient at the 30 mg dose level experienced SAE. On d 15 of the third cycle, the patient had numbness, weakness and limitation of motion on the left limbs. Acute cerebral infarction was diagnosed, and the patient was hospitalized for treatment and withdrawn from the study. The investigator considered the SAE was unlikely related to the study medicines.
Pharmacokinetics
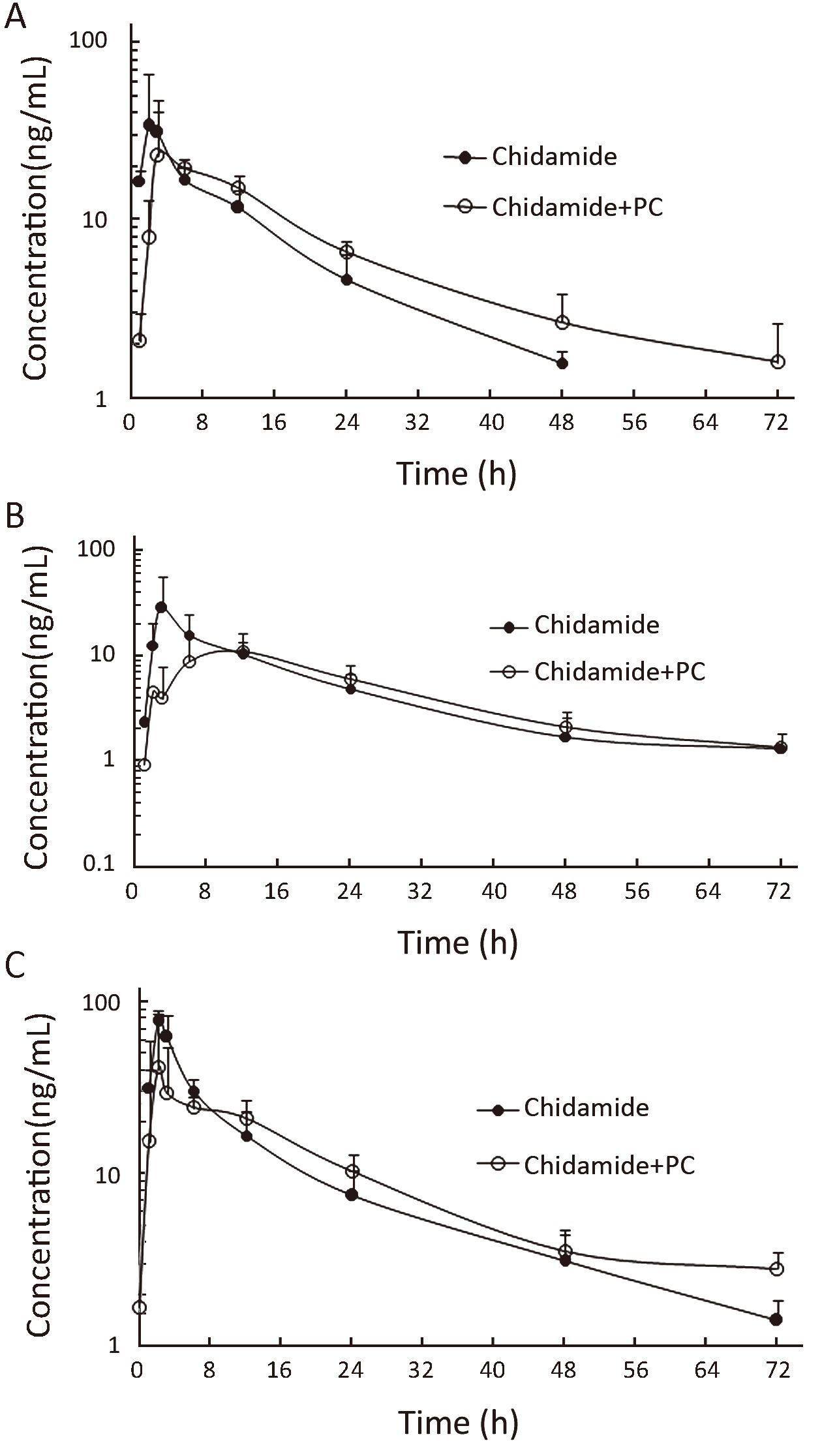
Plasma samples for pharmacokinetic studies were obtained from all the patients to detect potential relevant drug-drug interactions. Figure 1 shows the mean plasma concentrations of chidamide at different doses when administered alone and with paclitaxel and carboplatin. In general, no significant difference in chidamide concentration-time curve was observed before and after combination with paclitaxel/carboplatin. Furthermore, similar chidamide clearance was observed between single dose vs. combination with paclitaxel/carboplatin among all three doses in average (55.3±27.9 vs. 51.6±26.1 L/h, P=0.747). And there is no significant difference of chidamide values in Cmax (P=0.08) and AUC0-72 (P=0.97) by a paired t test between single and combination dosage.
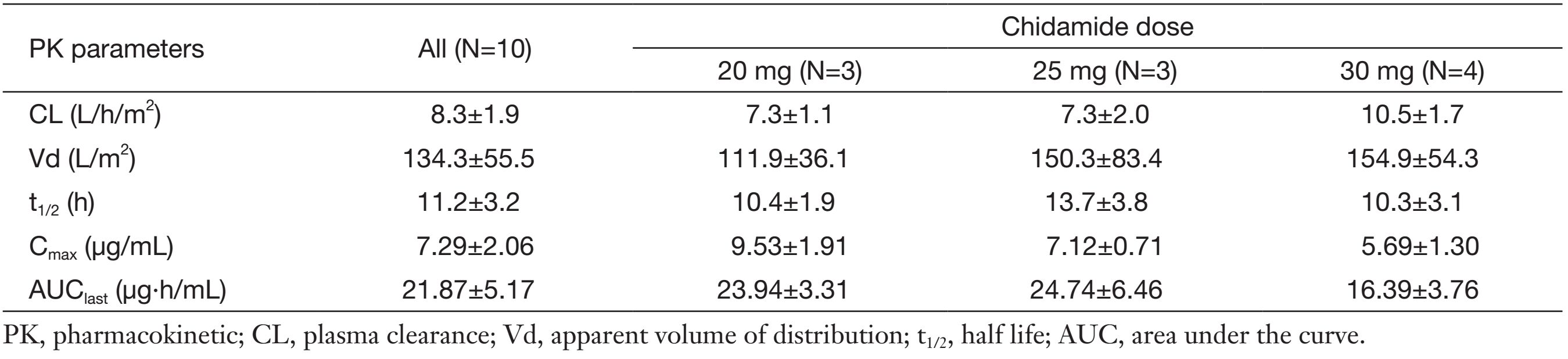
Major pharmacokinetic parameters of paclitaxel when combined with chidamide at the dose of 20, 25 and 30 mg are presented in Table 4. Similar paclitaxel pharmacokinetic estimates were seen when combined with chidamide at different doses (P>0.05), and these values are in agreement with reported values for comparable dose of single-agent paclitaxel administered as a 3-h i.v. infusion (16).
Full table
Efficacy
There are 8 patients were evaluable for tumor response since 2 patients in the 30 mg cohort withdrew from the study due to DLTs before response assessment. Confirmed partial response (PR) was observed in 1 patient in the 20 mg cohort, with a duration of 166 d. SD was seen in 4 patients: 1 in 20 mg cohort, 1 in 25 mg cohort, 2 in 30 mg cohort, and the duration of SD was 1+, 28+, 47 and 105 d, respectively ("+" denotes a censored value). Progressive disease (PD) was seen in 3 patients.
Five patients (3 in 20 mg cohort, 1 in 25 mg cohort and 1 in 30 mg cohort) had brain metastases prior to study entry. Two patients in the 20 mg cohort with brain lesions of 6-9 mm achieved intracranial complete response (CR) after 6-week treatment, which lasted for 12 weeks (data not shown).
Discussion
Chidamide represents a novel benzamide class HDAC inhibitor with unique mechanism of actions and significant orally single-agent activity against relapsed or refractory PTCL as single agent (14). This dose-escalation phase I study was designed and conducted to determine the safety and maximum tolerated dose (MTD) of chidamide in combination with paclitaxel and carboplatin to identify a regimen suitable for further phase II/III evaluation.
In this study, the most frequently reported AEs were myelosuppression, which were principally neutropenia, thrombocytopenia, leucopenia and anemia. All patients (100%) and 3 patients (30%) respectively experienced grade 3/4 neutropenia and thromcytopenia, which were more frequent than those in previous studies evaluating paclitaxel/carboplatin alone (17-19). It was probably due to the overlapping hematological toxicity of chidamide and paclitaxel/carboplatin. Consistent with the side effect profiles for paclitaxel-carboplatin or single chidamide, the combination treatment showed mild to moderate non-hematological toxicities without grade 3/4 AEs, which suggested little additive non-hematological toxicities in those patients to the regimen.
DLTs were documented in 2 patients from the 30 mg chidamide cohort with a total 4 patients enrolled, including 1 grade 4 thrombocytopenia, and 1 treatment delay due to grade 3 neutropenia and grade 2 thrombocytopenia. Therefore, DLT for chidamide combined with paclitaxel/carboplatin was identified as 30 mg, and no further patients were enrolled in this cohort. Although similar frequency and severity of AEs were represented between the 25 mg and 20 mg cohorts, all 3 patients in the 25 mg cohort had treatment discontinuation after cycle 2 due to the disease progression and investigator's discretion, which prevented this cohort from adequate evaluation for repeated administration. Based on these considerations and potential efficacy observed in the 20 mg cohort, 20 mg chidamide in combination with fixed dose of paclitaxel (175 mg/m2) and carboplatin (AUC=5 mg/mL/min) was recommended as phase II regimen for further evaluation.
Chidamide in combination with paclitaxel/carboplatin displayed similar pharmacokinetic properties to those from phase I study of single chidamide (8). Regardless of dose levels, no significant difference was seen in the plasma clearance (CL) of chidamide before and after combination with paclitaxel and carboplatin. We also investigated the potential effects of chidamide on the pharmacokinetics of paclitaxel: similar paclitaxel pharmacokinetic estimates were seen in co-administration with chidamide at different doses. Paclitaxel had a half life (t1/2) of 11.2±3.2 h and a CL of 8.3±1.9 L/h/m2 when administered at 175 mg/m2 in our study. It was favorably comparable with the pharmacokinetic parameters of paclitaxel (Bristol-Myers Squibb, USA) in the FDA database with a t1/2 of 9.0±4.9 h, and a CL of 10.5±6.4 L/h/m2 at the dose of 170-175 mg/m2, as well as with reported values for comparable dose of single-agent paclitaxel administered (16). These results suggested lack of significant drug-drug interaction between chidamide and paclitaxel during co-administration.
Five patients completed 3 or more cycles of treatment. One patient in the 20 mg cohort had confirmed PR. Of 5 patients with brain metastases, 2 from the 20 mg cohort had complete remission of brain tumors after 4-cycle treatment. Poor permeability across the blood brain barrier was reported for paclitaxel and carboplatin (20, 21), and intracranial control was rarely seen in patients treated with either of the agents (22). However, whether the observed antitumor activity for brain metastasis in this trial could be contributed to the addition of chidamide to the chemotherapy regimen needs further study to elucidate.
Conclusions
The results of this study on 10 patients with advanced NSCLC showed that the combination regimen of chidamide plus paclitaxel/carboplatin is generally tolerable, with the preliminary evidence of tumor control and no apparent chidamide-paclitaxel pharmacokinetic interactions. A phase II study has been performed to further evaluate the efficacy and safety of chidamide 20 mg BIW plus paclitaxel/carboplatin for the treatment of advanced NSCLC patients with wild-type EGFR genotype.
Acknowledgements
We gratefully acknowledge Zhiqiang Ning and Xianping Lu from Chipscreen Biosciences Ltd. for their contributions to this study.
Funding: This study was supported in part by grants from Chinese National Major Project for New Drug Innovation (2012ZX09303012-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [PubMed] DOI:10.3322/caac.v61:2
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [PubMed] DOI:10.3322/caac.21254
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48–58. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2012;346:92–8.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. [PubMed] DOI:10.1200/JCO.2007.15.0375
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50. [PubMed] DOI:10.1056/NEJMoa061884
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525–31. [PubMed] DOI:10.1016/S0140-6736(09)60569-9
- Dong M, Ning ZQ, Xing PY, et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol 2012;69:1413–22. [PubMed] DOI:10.1007/s00280-012-1847-5
- Gong K, Xie J, Yi H, et al. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J 2012;443:735–46. [PubMed] DOI:10.1042/BJ20111685
- Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 2012;69:901–9. [PubMed] DOI:10.1007/s00280-011-1766-x
- Yao Y, Zhou J, Wang L, et al. Increased PRAME-specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS One 2013;8:e70522. [PubMed] DOI:10.1371/journal.pone.0070522
- Pan DS, Yang QJ, Fu X, et al. Discovery of an orally active subtype-selective HDAC inhibitor, chidamide, as an epigenetic modulator for cancer treatment. Med Chem Comm 2014;5:1789–96. DOI:10.1039/C4MD00350K
- Zhou Y, Pan DS, Shan S, et al. Non-toxic dose chidamide synergistically enhances platinum-induced DNA damage responses and apoptosis in non-small-cell lung cancer cells. Biomed Pharmacother 2014;68:483–91. [PubMed] DOI:10.1016/j.biopha.2014.03.011
- Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol 2015;26:1766–71. [PubMed] DOI:10.1093/annonc/mdv237
- Gu R, Liu T, Zhu X, et al. Development and validation of a sensitive HPLC-MS/MS method for determination of chidamide (epidaza), a new benzamide class of selective histone deacetylase inhibitor, in human plasma and its clinical application. J Chromatogr B Analyt Technol Biomed Life Sci 2015;1000:181–6. DOI:10.1016/j.jchromb.2015.07.001
- Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995;13:180–90. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [PubMed] DOI:10.1056/NEJMoa0810699
- Lara PN Jr, Douillard JY, Nakagawa K, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2965–71. [PubMed] DOI:10.1200/JCO.2011.35.0660
- Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2667–74. [PubMed] DOI:10.1200/JCO.2010.32.8971
- Glantz MJ, Choy H, Kearns CM, et al. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst 1995;87:1077–81. [PubMed] DOI:10.1093/jnci/87.14.1077
- Jacobs SS, Fox E, Dennie C, et al. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Cancer Res 2005;11:1669–74. [PubMed] DOI:10.1158/1078-0432.CCR-04-1807
- Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol 2015;33:3475–84. [PubMed] DOI:10.1200/JCO.2015.60.9503
