Detection of ALK translocation in non-small cell lung carcinoma (NSCLC) and its clinicopathological significance using the Ventana immunohistochemical staining method: a single-center large-scale investigation of 1, 504 Chinese Han patients
Lin Yang1, Yun Ling1, Lei Guo1, Di Ma2, Xuemin Xue1, Bingning Wang1, Junling Li2, Jianming Ying1
Contributions: (I) Conception and design: L Yang; (II) Administrative support: Y Ling, L Guo, B Wang, J Li, J Ying; (III) Provision of study materials or patients: L Yang; (IV) Collection and assembly of data: L Yang, D Ma; (V) Data analysis and interpretation: X Xue; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Abstract
Objective: The novel fully automated immunohistochemistry (IHC) assay-Ventana anaplastic lymphoma kinase (ALK)-D5F3 for screening ALK rearrangements has been approved by China’s Food and Drug Administration in 2013, our previous study disclosed a highly specificity and sensitivity nearly 100%, and its efficacy needs to be evaluated in a large cohort of primary lung adenocarcinoma patients, and to compare clinicopathological features with ALK (+) and ALK (-) lung adenocarcinoma.
Methods: A total of 1,504 consecutive surgical lung adenocarcinoma cases of Chinese Han population were collected and re-diagnosed according to the 2011 multidisciplinary classification of lung adenocarcinoma. Fully automated Ventana ALK-D5F3 IHC staining with a binary scoring was adopted to evaluate staining and correlated with clinicopathological characters, including age, sex, differentiation degree, histological subtype, lymph node metastasis, and clinical staging. ALK (+) patients were followed-up, and targeted therapy of ALK-inhibitors was adopted and observed in patients with stage IV according to the NCCN guideline.
Results: ALK positive adenocarcinomas were identified in 6.6% of the surgically resected 1,504 NSCLCs, and significantly younger than the negative group (P<0.05).Mucinous adenocarcinoma (28.2%) was determined to be predominant in ALK (+) cases, followed by the solid type (11.7%), specific type (6.8%), papillary type (5.6%), acinar type (5.5%), and lepidic type (3.1%), and the differences were statistically significant (χ2=42.011, P<0.05). ALK (+) adenocarcinoma with lymph node metastasis (10.8%) were significantly higher than that without lymph node metastasis (4.5%) (χ2=19.809, P<0.05); and ALK (+) in phase IV (20%) was significantly higher than phase III (12.9%), phase II (4.2%), phase I (4.5%), and phase 0 (0) (χ2=36.068, P<0.05). Multivariate logistic regression disclosed that patient age, AJCC staging, and histological mucinous subtype were correlated with ALK positive staining (OR=0.959, 1.578, 5.036, respectively). Sixty eight patients had followed-up results, five patients out of which primarily diagnosed or progressed into Stage IV benefited well from targeted therapy with Crizotinib.
Conclusions: The ALK fusion protein was seen in 6.6% Chinese NSCLC patients, and mostly seen in younger, clinically higher staging, mucinous and solid predominant adenocarcinoma. Clinical trials in patients of Stage IV confirmed that ALK-D5F3 Ventana IHC is serviceable in screening ALK-positive candidates for molecular targeted therapy.
Keywords: Anaplastic lymphoma kinase (ALK) rearrangements; fully automated immunohistochemistry (IHC); clinicopathological analysis; targeted therapy
Submitted Jul 13, 2015. Accepted for publication Jan 06, 2016.
doi: 10.21147/j.issn.1000-9604.2016.05.04
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers, which is the leading cause of cancer deaths worldwide (1). Although conventional chemotherapy remains as the main treatment regimen for the majority of advanced NSCLC patients, the identification of specific genetic oncogenic abnormalities has led to the development of new targeted therapies within a subset of NSCLS patients (2). Fusion of the echinoderm microtubule-associated protein like-4 (EML4) and anaplastic lymphoma kinase (ALK), which accounts for 2-11% of NSCLC cases, represents another distinct mechanism of driver mutation in NSCLC, following EGFR mutation (3-5). Several clinical trials have demonstrated the remarkable efficacy of Crizotinib for the treatment of locally advanced or metastatic NSCLC patients who harbor ALK rearrangements (6,7). Three methods have been applied for discriminating ALK (+) NSCLC, including fluorescent in situ hybridization (FISH), quantitative real-time polymerase chain reaction (qRT-PCR), and a novel fully automated immunohistochemistry (IHC), Ventana ALK-D5F3 IHC (8,9).
Our previous study disclosed the novel fully automated Ventana ALK-D5F3 IHC is a highly sensitive (100%) and specific (98%) method for the detection of ALK rearrangements in primary lung adenocarcinoma and is especially suitable for paraffin-embedded tissue (10-12). We also found positive responses to Crizotinib in patients with ALK positive lung adenocarcinoma who tested Ventana IHC-positive and FISH-negative (in press), and surprisingly found a novel fusion gene in ALK Break-Apart FISH-Negative but Ventana-IHC positive lung Adenocarcinoma (13).However, the screening efficacy of Ventana-D5F3 IHC method on large-scale Chinese Han population needs to be further evaluated, and few literature involves in it.
Besides, in terms of the clinicopathological characteristics of ALK (+) NSCLC, several studies have investigated the predictive value of pathological and morphological features in detecting ALK-rearranged tumors (14), and few studies used the new International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) classification in the comparative analysis ALK (+) lung adenocarcinoma (15,16). So, it is necessary to observe the clinical and pathological characteristics of ALK (+) lung adenocarcinoma by application of 2011 IASLC/ATS/ERS new classification of lung adenocarcinoma.
The aim of this study was: (I) to screen and detect ALK rearrangements by Ventana ALK-D5F3 IHC in a large cohort of primary lung adenocarcinoma patients from Chinese Han population; (II) to compare clinicopathological features between ALK (+) and ALK (−) lung adenocarcinoma based on the 2011 IASLC/ATS/ERS classification and other clinicopathological characters.
Materials and methods
Case selection and histological analysis
This study was a retrospective study, which had been approved by the hospital ethics committee to exempt patient’s informed consent. All pathologically diagnosed pulmonary adenocarcinoma (or mixed adenocarcinoma) surgical resection specimens were consecutively collected from the Department of Pathology of the Cancer Hospital, Chinese Academy of Medical Sciences from July 2013 to September 2014. Clinicopathological data were extracted from medical archives, including the patient’s age, sex, tumor size, lymph node metastasis, AJCC 7th clinical stage, available radiologic images, surgical findings, and treatment by Crizotinib. Histological subtype and differentiation degree were reviewed and distinguished by two experienced pathologists according to the 2011 IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma (Kappa=0.7). A total of 1, 504 cases were classified according to dominant components, which included adenocarcinoma in situ (15 cases, 1%), minimally invasive adenocarcinoma (6 cases, 0.4%), lepidic adenocarcinoma (140 cases, 9.3%), papillary adenocarcinoma (231 cases, 15.4%), acinar adenocarcinoma (850 cases, 56.5%), solid adenocarcinoma with mucin produced (179 cases, 11.9%), mucinous adenocarcinoma (39 cases, 2.6%), micropapillary adenocarcinoma (13 cases, 0.9%), adenosquamous carcinoma (18 cases, 1.2%), enteric type adenocarcinoma (3 cases, 0.2%), mixed adenocarcinoma and small cell carcinoma (adeno-SCLCs, 4 cases, 0.3%), and sarcomatoid carcinoma (6 cases, 0.4%). For statistical purposes, special type was created, which consisted of a total of 44 cases, including 18 adenosquamous carcinomas, 13 micropapillary adenocarcinomas, 6 sarcomatoid carcinomas, 4 mixed adeno-SCLCs, and 3 enteric adenocarcinomas. The lepidic pattern comprised a total of 161 cases, including 140 lepidic adenocarcinomas, 15 adenocarcinomas in situ, and 6 minimally invasive adenocarcinomas.
Ventana IHC staining and scoring
Preprocessing procedures of specimens were as follows: surgically resected tissues were fixed in 10% neutral formalin for 6-48 h at 10 times the volume of the tissue liquid, and then embedded in paraffin. Two consecutive 4-μm-thick sections were cut; one section was used in ALK-D5F3 IHC analysis, and the other for routine negative control staining for a matched rabbit monoclonal negative Ig antibody. All sections were heated to 62 ℃, and then subjected to the fully automated IHC assay developed by Ventana using the pre-diluted Ventana anti-ALK (D5F3) rabbit monoclonal primary antibody, together with the Optiview DAB IHC detection kit and Optiview Amplification kit on the Benchmark XT stainer. According to the manufacture’s scoring algorithm, a binary scoring system (positive or negative for ALK status) was adopted for the evaluation of staining results. The presence of strong granular cytoplasmic staining in tumor cells (any percentage of positive tumor cells) was deemed ALK-positive, whereas the absence of strong granular cytoplasmic staining in tumor cells was designated as ALK-negative (Figure 1). Negative quality control sections were first evaluated to remain unstained, and false positive staining should be excluded for alveolar macrophage cytoplasm, neurogenic cells (nerve and ganglion cells), normal mucosa and alveolar epithelial cell coloring, necrotic material non-specific coloring etc.
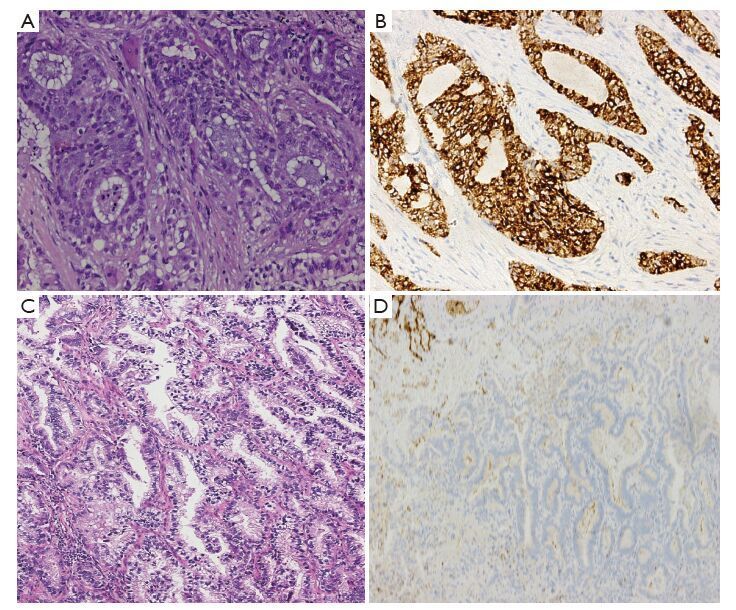
Follow-up of ALK (+) patients
All patients were consecutively enrolled in this study from July 2013 to September 2014, and a follow-up for the ALK (+) patients was made from July 2013 to April 2015, with a period of one to twenty one months.
Statistical methods
We used independent sample t test , Chi-square test, or Fisher’s exact test to compare frequency of clinicopathological characters between ALK (+) and ALK (−) groups. Multivariate logistic regression was also performed to detect correlation of ALK positive staining with clinicopathological characters. The statistical analyses were conducted using SPSS version 17.0 software (SPSS, Chicago, IL, USA), and statistical significance was set as P<0.05.
Results
Positive rate of ALK and clinically related features
A total of 1, 504 sections from primary lung adenocarcinoma patients were available for analysis, of which 100 (6.6%) were identified as ALK-positive by ALK-D5F3 Ventana IHC, including 47 males and 53 females, with the average age of 54 years. Among these, 3 cases were within the second decade of life (accounting for 3%), 7 cases the third decade (7%), 21 cases the forth decade (21%), 35 cases the fifth decade (35%), 26 cases the sixth decade (26%), and 8 cases the eighth decade (8%). Smoking history and family history of cancer were retrieved from medical archives, 35% cases has a history of smoking (35/100), 25% (25/100) with family history of cancer, including 10 lung cancer family history (40%, 10/25).
Correlation between ALK-positive expression and clinicopathological characteristics
The associations between ALK positive expression and clinicopathological characteristics are listed in Table 1, and those cases without complete records of lymph node metastasis [8 ALK (+) out of 101, 8/101], T staging (4/36) or clinical staging (4/81) were excluded in the statistics. The ALK-positive group was significantly younger than the negative group (P<0.05), whereas no significant differences in gender were observed (P>0.05). In terms of histological types, the rate of positive expression in mucinous adenocarcinoma (28.2%) was the highest, followed by the solid type (11.7%), specific type (6.8%), papillary type (5.6%), acinar type (5.5%), and lepidic type (3.1%), and the differences were statistically significant (χ2=42.011, P<0.05). In terms of differentiation degree, the rate of positive expression in poorly differentiated adenocarcinoma (9.8%) was significantly higher than that in moderately differentiated adenocarcinoma (7.5%) and in highly differentiated adenocarcinoma (3.2%) (χ2=11.618, P<0.05). The rate of positive expression in adenocarcinoma with lymph node metastasis (10.8%) was significantly higher than that in adenocarcinomas without lymph node metastasis (4.5%) (χ2=19.809, P<0.05). In terms of clinical stage (AJCC 7th ed.), the rate of positive expression of phase IV (20%) was significantly higher than that in phase III (12.9%), phase II (4.2%), phase I (4.5%), and phase 0 (0) (χ2=36.068, P<0.05), whereas for the T stage, no significant differences in the rate of positive expression were observed among T4 (10.9%), T3 (12.1%), T2 (6.4%), T1 (5.8%), and Tis (0) (χ2=8.020, P=0.09) (Table 1).
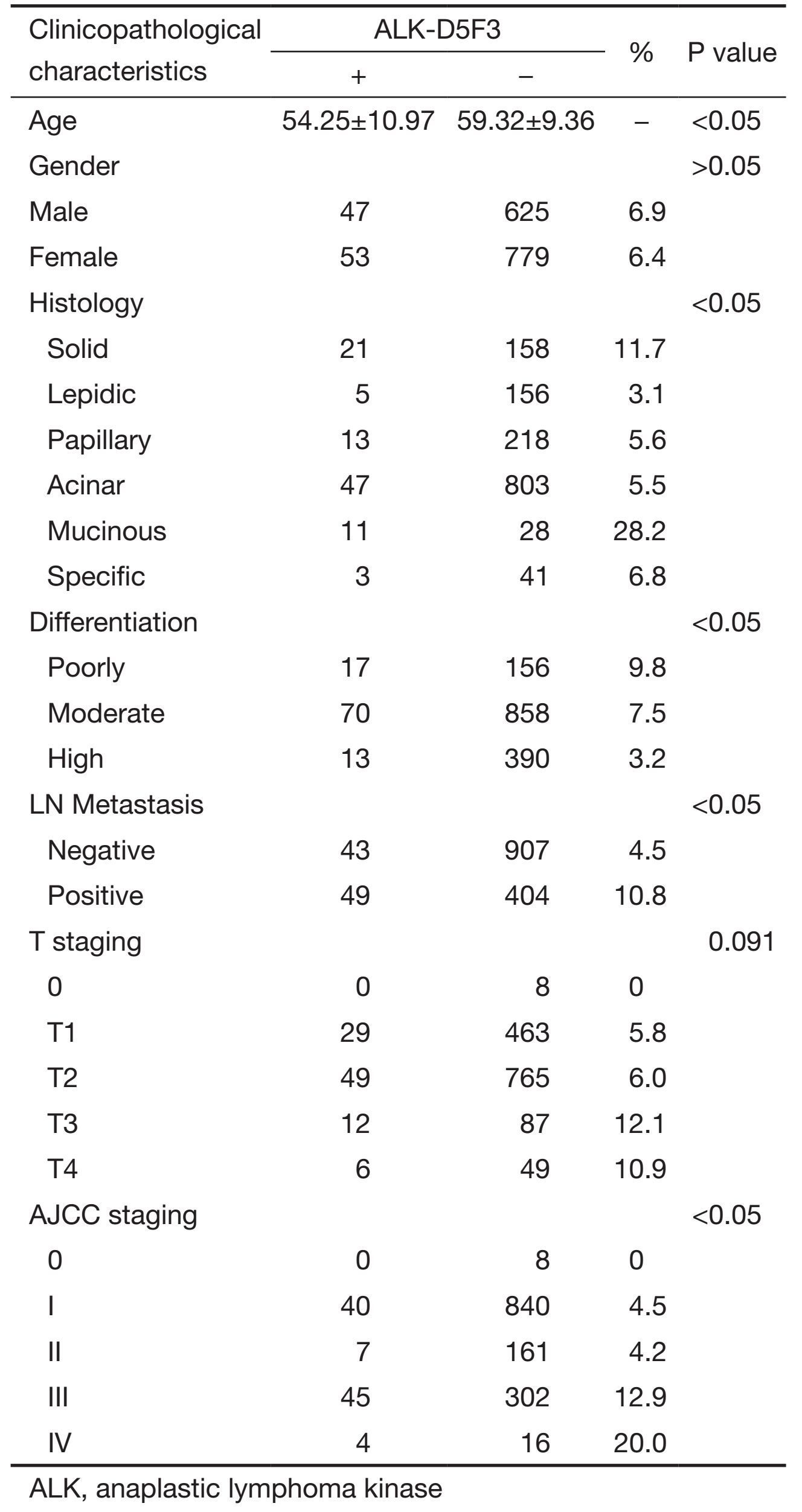
Full table
As for correlation of ALK positive staining with clinicopathological characters, we conducted a multivariate logistic regression simultaneously. The ALK staining status was deemed as a dependent variable, and clinical stage, lymph node metastasis, differentiation degree and histological type were independent variables. Besides, the histological types were set dummy variable with the reference of solid subtype. It showed in the multivariate logistic regression that the more advanced staging, the higher possibility of ALK-positive expression; the mucinous adenocarcinoma was higher than that of the solid subtype, and no difference was found among solid subtype, lepidic, papillary, specific type and acinar subtype. In terms of lymph node metastasis, we found that ALK-positive expression was significantly higher in lymph node metastatic adenocarcinoma than that of those without lymph node metastasis in a univariate analysis, although no statistical significance was observed in multivariate factor analysis. Similarly, the correlation between the differentiation degree and the positive expression of ALK was same as the lymph node metastasis. On the contrary, patient age, AJCC staging, and histological type were all statistically significant both in the univariate and multivariate analyses, suggesting that these were independent relevant factors of ALK-positive expression (Table 2).
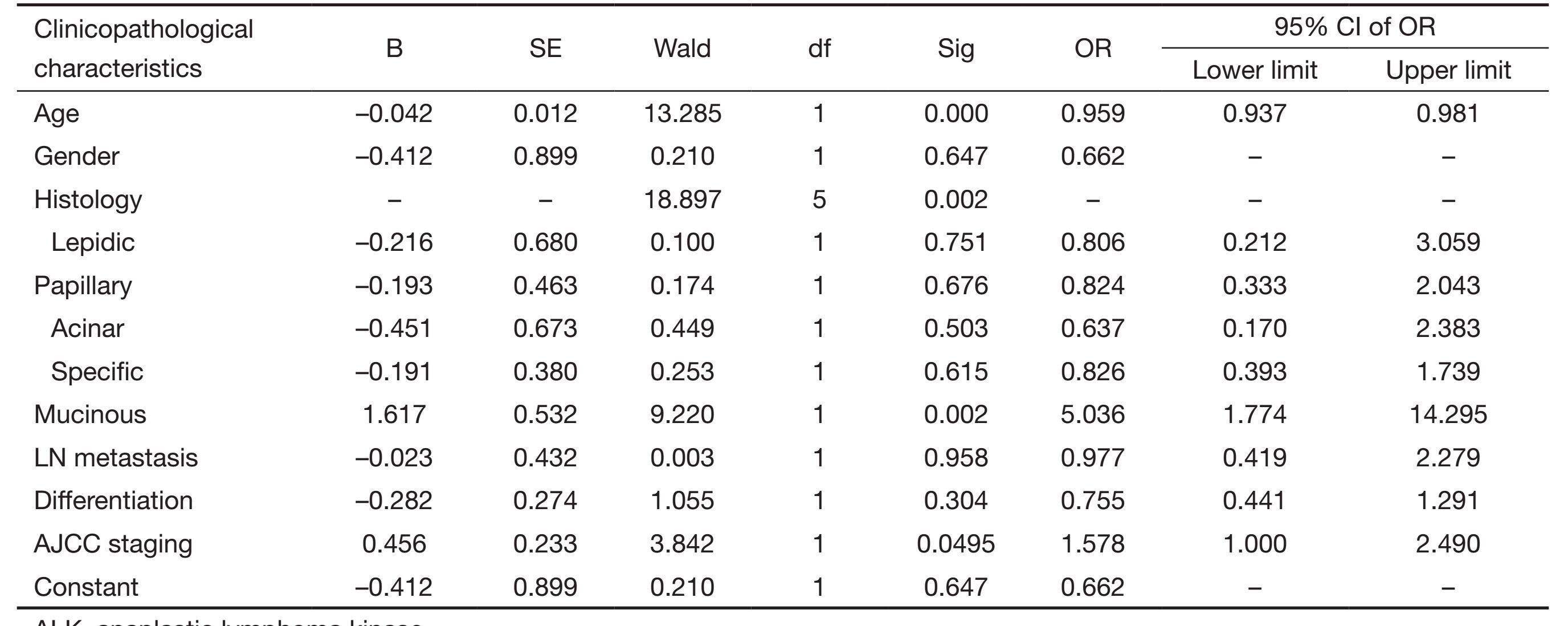
Full table
Follow-up and treatment of Stage IV ALK-positive patients
All patients were consecutively enrolled in this study from July 2013 to September 2014, and we conducted a follow-up for the 100 ALK (+) patients from July 2013 to April 2015, with a period of one to twenty one months. Four advanced cases of Stage IV were ALK-D5F3 positive at the beginning of follow-up, with one other ALK (+) case of Stage IIIa progression into Stage IV during the follow-up period. All of the above five advanced or metastatic cases were treated by ALK-inhibitor targeted therapy according to the guideline (9), and received partial response (PR) or stable disease (SD), except for case number 4, who died due to posterior meninges metastasis (Table 3,Figure 2). The other 95 ALK (+) patients were Stage I-Stage III, and were not suitable for targeted therapy of ALK-inhibitor.
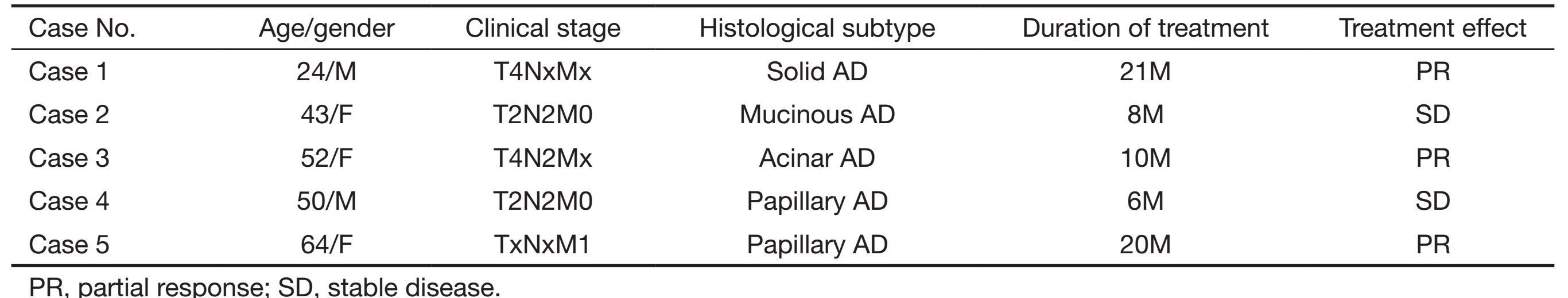
Full table
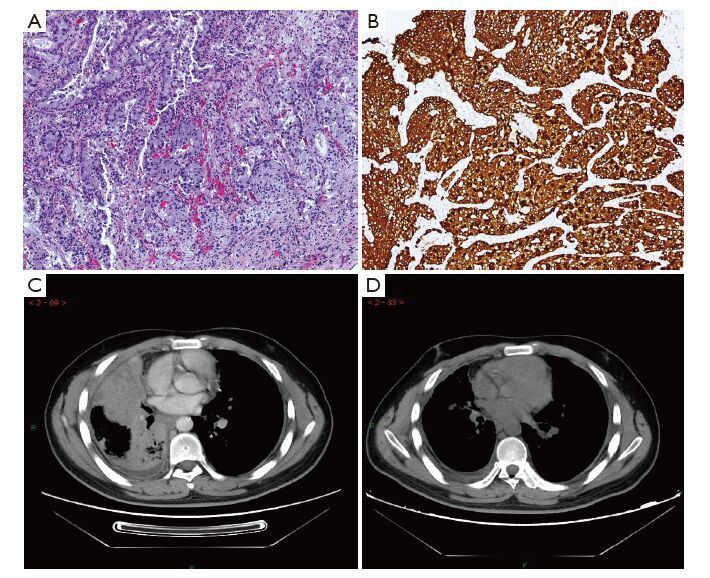
Discussion
ALK fusion is one of the newest tyrosine-kinase targets in NSCLC. It is aberrantly activated due to a chromosomal rearrangement that leads to the expression of an oncogenic fusion kinase, ALK fusion protein, which can be beneficial for ALK inhibitor treatment (3). ALK fusion NSCLC is diagnosed when the ALK fusion gene or protein is detected by FISH, RT-PCR, and specific immunohistochemistry (9).FISH has been utilized for the detection of ALK fusion gene, particularly in determined whether the patient will benefit from ALK-targeted therapy. However, extensive research on the applicability of the ALK fusion protein has determined that some FISH results are false negative, which in turn dissuades the oncologist to administer targeted therapy (17). According to our previous studies, the Ventana ALK fully automated IHC detection method has been determined to be of 100% sensitivity and almost 100% specificity (10,12), and it has also been determined to be more useful in lower cost, efficacy for detection and interpretation, and suitability for clinical screening and diagnosis in large study populations, especially in paraffin-embedded tissues (9). Using the Ventana method in this study, we successfully identified 100 ALK-positive cases from 1, 504 consecutive surgical specimens of primary lung adenocarcinoma in one single center from July 2013 to September 2014. The center was determined to have an ALK (+) adenocarcinoma incidence rate of 6.6%, which is within the range reported in literatures in Western or other Asian Countries (18-20), and consistent with our smaller sample detected by FISH before (10,12). The results indicated that Ventana-D5F3 IHC is a valid alternative method to detect ALK (+) NSCLC.
In NSCLC, ALK rearrangement is associated with distinct clinicopathologic features, including young age at onset and adenocarcinoma histology in patients with a history of never smoking or light smoking and no indication of an EGFR mutation (17-19). Generally, most of the clinicopathologic features of ALK-positive patients in the present study were similar to that of previous studies, including younger age and high grade clinical staging (18-20). However, we found differentiation degree and lymph node metastasis were correlated with ALK-positive expression in a univariate analysis, while no statistical significance was observed in the multivariate factor analysis. Further study is needed to disclose the correlation between ALK positive staining with lymph node metastasis.
In clinicopathological practice, we know that lung adenocarcinoma is highly variable and shows extensive heterogeneity (21). The 2011 IASLC/ATS/ERS multidisciplinary adenocarcinoma classification and the 2015 WHO classification of lung adenocarcinoma divides invasive lung adenocarcinoma into five major different histological subtypes and other specific subtypes (15). Whether different histological subtypes of adenocarcinoma are correlated with the positive expression of ALK? In other words, are there any differences in ALK-positive expression based on various histological morphology? The general perception is that poorly differentiated adenocarcinoma, accompanied by trabecular structure, extracellular mucus secretion, or signet ring cells show a higher positive expression of ALK fusion gene or protein (14, 19, 22). In the present study, we observed that the ALK-positive rate in mucinous adenocarcinoma was higher than that of solid predominant adenocarcinoma, although no significance was observed between solid predominant adenocarcinoma and other subtypes, including lepidic, papillary, acinar, micropapillary, adenosquamous carcinoma. To our knowledge, this is the first report that examined the correlation between ALK-positive expression and the 2011 multidisciplinary lung adenocarcinoma classification by Ventana IHC in large-scale screening of surgical specimens. The present study also showed that the ALK fusion protein is present in almost all kinds of adenocarcinoma, and such characters as “younger, mucinous adenocarcinoma, or containing signet ring cell component” are not specific or exclusively positive for ALK fusion gene or protein. Thus, routine screening of ALK (+) cases from NSCLC is very important in clinical practice, and the Ventana-IHC is the feasible screening method especially for paraffin tissue blocks. These features have been used as the theoretical bases for the CFDA approval of Ventana-IHC in screening and diagnosing ALK (+) NSCLC for Crizotinib targeted therapy (9).
In summary, the present study screened ALK fusion protein by Ventana-IHC in a large cohort of Chinese Han Population, and established the correlation with ALK fusion protein expression and clinicopathological characters mainly based on the 2011 multidisciplinary classification of lung adenocarcinoma for the first time.
Conclusions
The ALK fusion protein was seen in 6.6% Chinese NSCLC patients, and mostly seen in younger, clinically higher staging, mucinous and solid predominant adenocarcinoma. Small-scale clinical trials indicated feasibility of ALK-positive candidates for molecular targeted therapy, and further study will be explored in efficacy of Ventana-IHC in determining targeted therapy of ALK-inhibitors.
Acknowledgements
We thank Let Pub for its linguistic assistance during the preparation of this manuscript.
Funding: The study was supported by the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332015060).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- International Agency for Research on Cancer. Globocan 2012:estimated cancer incidence, mortality and prevalence worldwide in 2012. Available online: http://globocan.iarc.fr
- Bittner N, Ostoros G, Géczi L. New treatment options for lung adenocarcinoma--in view of molecular background. Pathol Oncol Res 2014;20:11–25. [PubMed] DOI:10.1007/s12253-013-9719-9
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13–7. [PubMed] DOI:10.1097/JTO.0b013e31815e8b60
- Fu S, Wang F, Shao Q, et al. Detection of EML4-ALK fusion gene in Chinese non-small cell lung cancer by using a sensitive quantitative real-time reverse transcriptase PCR technique. Appl Immunohistochem Mol Morphol 2015;23:245–54. [PubMed] DOI:10.1097/PDM.0000000000000038
- Shi Y, Sun Y. Medical management of lung cancer: Experience in China. Thorac Cancer 2015;6:10–6. [PubMed] DOI:10.1111/tca.2015.6.issue-1
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881–8. [PubMed] DOI:10.1200/JCO.2014.59.0539
- Rossi A, Maione P, Sacco PC, et al. ALK inhibitors and advanced non-small cell lung cancer (review). Int J Oncol 2014;45:499–508. [PubMed]
- Teixidó C, Karachaliou N, Peg V, et al. Concordance of IHC, FISH and RT-PCR for EML4-ALK rearrangements. Transl Lung Cancer Res 2014;3:70–4. [PubMed] DOI:10.3978/j.issn.2218-6751.2014.02.02
- Zhang X, Lu S, Zhang L, et al. The Chinese expert consensus opinion of the diagnosis of ALK-positive NSCLC (2013 version). Chin J Pathol 2013;42:402–6.
- Guo L, Liu X, Qiu T, et al. ALK fusion gene assessment by fully automatic immunohistochemistry in non-small cell lung cancer. Zhonghua Bing Li Xue Za Zhi 2014;43:95–8. [PubMed]
- Shan L, Lian F, Guo L, et al. Combination of conventional immunohistochemistry and qRT-PCR to detect ALK rearrangement. Diagn Pathol 2014;9:3. [PubMed] DOI:10.1186/1746-1596-9-3
- Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol 2013;24:2589–93. [PubMed] DOI:10.1093/annonc/mdt295
- Shan L, Jiang P, Xu F, et al. BIRC6-ALK, a Novel Fusion Gene in ALK Break-Apart FISH-Negative Lung Adenocarcinoma, Responds to Crizotinib. J Thorac Oncol 2015;10:e37–9. [PubMed] DOI:10.1097/JTO.0000000000000467
- Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010;63:1066–70. [PubMed] DOI:10.1136/jcp.2010.081166
- Tang Y, He Z, Zhu Q, et al. The 2011 IASLC/ATS/ERS pulmonary adenocarcinoma classification: a landmark in personalized medicine for lung cancer management. J Thorac Dis 2014;6:S589–96. [PubMed]
- Lee HJ, Lee CH, Jeong YJ, et al. IASLC/ATS/ERS International Multidisciplinary Classification of Lung Adenocarcinoma: novel concepts and radiologic implications. J Thorac Imaging 2012;27:340–53. [PubMed] DOI:10.1097/RTI.0b013e3182688d62
- Li Y, Pan Y, Wang R, et al. ALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCR. PLoS One 2013;8:e69016. [PubMed] DOI:10.1371/journal.pone.0069016
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–53. [PubMed] DOI:10.1200/JCO.2009.22.6993
- Fan L, Feng Y, Wan H, et al. Clinicopathological and demographical characteristics of non-small cell lung cancer patients with ALK rearrangements: a systematic review and meta-analysis. PLoS One 2014;9:e100866. [PubMed] DOI:10.1371/journal.pone.0100866
- Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011;35:1226–34. [PubMed] DOI:10.1097/PAS.0b013e3182233e06
- Hensing T, Chawla A, Batra R, et al. A personalized treatment for lung cancer: molecular pathways, targeted therapies, and genomic characterization. Adv Exp Med Biol 2014;799:85–117. [PubMed] DOI:10.1007/978-1-4614-8778-4
- Wang J, Cai Y, Dong Y, et al. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS One 2014;9:e101551. [PubMed] DOI:10.1371/journal.pone.0101551
