Comparison of lymph node number and prognosis in gastric cancer patients with perigastric lymph nodes retrieved by surgeons and pathologists
Lixin Jiang, Zengwu Yao, Yifei Zhang, Jinchen Hu, Dawei Zhao, Huiyuan Zhai, Xixun Wang, Zhenbin Zhang, Dong Wang
Abstract
Objective: To compare the numbers of positive and total lymph nodes and prognosis in gastric cancer patients whose perigastric lymph node retrieval was performed by surgeons and pathologists.
Methods: We conducted a retrospective analysis of clinical and follow-up data from 1, 056 patients who underwent gastric cancer D2 radical lymph node resection between January 2008 and December 2010 in the Gastrointestinal Surgery Department of Yantai Yuhuangding Hospital. The follow-up ended in December 2015. Patients were divided into two groups according to the specialty of physicians who performed the postoperative perigastric lymph node retrieval: the surgeon group (475 cases) and the pathologist group (581 cases). The numbers of positive and total perigastric lymph nodes and the 3- and 5-year survival were compared between gastric cancer patients in the two groups overall and stratified by TNM stage (the 7th Edition of the American Joint Committee on Cancer).
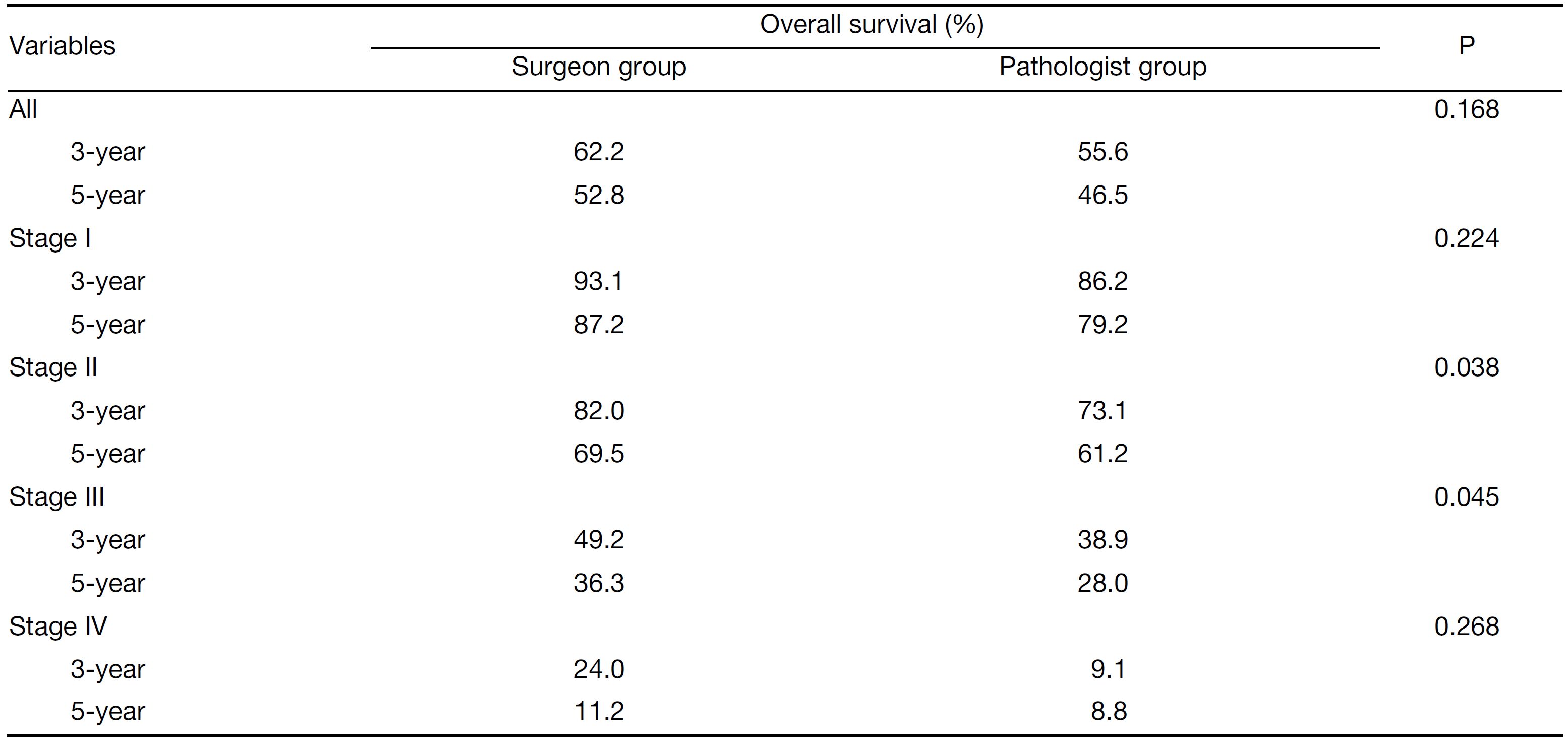
Results: Overall, the numbers of positive and total lymph nodes were significantly higher in the surgeon group than in the pathologist group (6.53±4.07 vs. 4.09±3.70, P=0.021; 29.64±11.50 vs. 20.71±8.56, P<0.001). Further analysis showed that the total number of lymph nodes in stage I patients (19.40±9.62 vs. 15.45±8.59, P=0.011) and the numbers of positive and total lymph nodes in stage II (1.38±1.08 vs. 0.87±1.55, P=0.031; 25.35±10.80 vs. 16.75±8.56, P<0.001) and stage III patients (8.11±6.91 vs. 6.66±5.12, P=0.026; 32.34±12.55 vs. 25.45±8.31, P<0.001) were significantly higher in the surgeon group than in the pathologist group. The survival analysis showed that the 3- and 5-year survival of stage II and III patients was significantly higher in the surgeon group than in the pathologist group (82.0% vs. 73.1%, 69.5% vs. 61.2%, P=0.038; 49.2% vs. 38.9%, 36.3% vs. 28.0%; P=0.045).
Conclusions: Compared with retrieval performed by pathologists, postoperative perigastric lymph node retrieval performed by surgeons was associated with significant increase in the total lymph node number of stage I patients, the numbers of positive and total lymph nodes of stage II and III patients, and the survival of stage II and stage III gastric cancer patients.
Keywords: Gastric cancer; surgeon and pathologist; lymph node retrieval; prognosis
Submitted Jul 10, 2016. Accepted for publication Oct 09, 2016.
doi: 10.21147/j.issn.1000-9604.2016.05.06
Introduction
Gastric cancer is one of the most common neoplasms and causes millions of deaths worldwide each year. This malignancy is one of the main causes of death in many developing countries, especially in China, where it is the cancer with the second highest annual incidence and the third leading cause of cancer-related mortality (1-4). Combined treatment, including surgery, perioperative radiotherapy and chemotherapy, has been recommended for the treatment of gastric cancer, and gastric cancer surgery has gone through several stages of development, from D1 lymph node dissection to D2 lymph node dissection, followed by extended D2 lymph node dissection (5-9) and finally the standard D2 lymph node dissection which is used at present (10,11). However, lymph node dissection for gastric cancer is difficult and controversial, and has been identified as one of the important prognostic factors in gastric cancer patients. The application of lymph node dissection for gastric cancer is in accordance with the standard of evidence-based medicine and principles of maximizing survival and minimizing mortality in patients. In the past, American and European surgeons routinely performed D1 or D1 plus lymph node dissection combined with postoperative radiotherapy and chemotherapy (12,13) to treat gastric cancer due to poor surgical skills and obesity in patients. Nowadays, standard D2 lymph node dissection has become a mainstream surgical technique in Asia, especially in China, Japan and South Korea, and has been accepted gradually by European and American surgeons (14). According to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging system for gastric cancer, the number of positive lymph nodes (15) is considered to be an important factor influencing gastric cancer staging. Thus, the method for postoperative lymph node retrieval may be a key factor in gastric cancer staging. In this study, we compared the numbers of positive and total lymph nodes and prognosis in gastric cancer patients whose perigastric lymph node retrieval was performed by surgeons and pathologists respectively.
Materials and methods
Data collection
Data were derived from Yantai Yuhuangding Hospital, the Affiliated Hospital of Qingdao University. We enrolled 1, 107 patients who had undergone D2 radical lymph node resection for gastric cancer from January 2008 to December 2011. Ultimately, data from 1, 056 cases including 654 males and 402 females were analyzed because 28 cases were lost to follow-up and 23 cases died from non-gastric cancer-related diseases. The median age of these patients was 58 (range, 31-89) years old. All operations were performed by experienced gastrointestinal surgeons.
The patients were divided into two groups according to the specialty of physicians who performed the postoperative perigastric lymph node retrieval: the surgeon group (475 cases) and the pathologist group (581 cases). For patients in the surgeon group, the surgeons sequentially retrieved lymph nodes postoperatively within half an hour according to lymph node station (Figure 1) and then submitted the lymph node specimens to the Pathology Department for further examination. For patients in the pathologist group, the pathologists retrieved lymph nodes postoperatively. TNM staging was determined according to the Union for International Cancer Control (UICC)/AJCC guidelines (2010, 7th Edition). This research protocol was approved by the Ethics Committee of Yantai Yuhuangding Hospital, the Affiliated Hospital of Qingdao University.
Follow-up
Follow-up was carried out by outpatient review and telephone interviews, including obtaining medical history, performing physical examinations and determining the presence of tumor markers every 3 to 6 months over the first 3 postoperative years, every 6 months during the third, fourth and fifth postoperative years, and every year after 5 postoperative years. Abdominal CT scans or gastric endoscopy examinations were carried out when necessary according to clinical judgement. The follow-up interval was from the operation date until October 2015, and the median follow-up duration was 54 months. The longest time is 94 months, and the shortest time is 1 month.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA). We compared the differences between two groups using t-test, χ2 or Fisher's exact probability tests. The Kaplan-Meier method was used to calculate the survival rates, and log-rank tests were used to test for survival differences between the two groups. Two-tailed P<0.05 was considered statistically significant in all tests.
Results
General information of patients
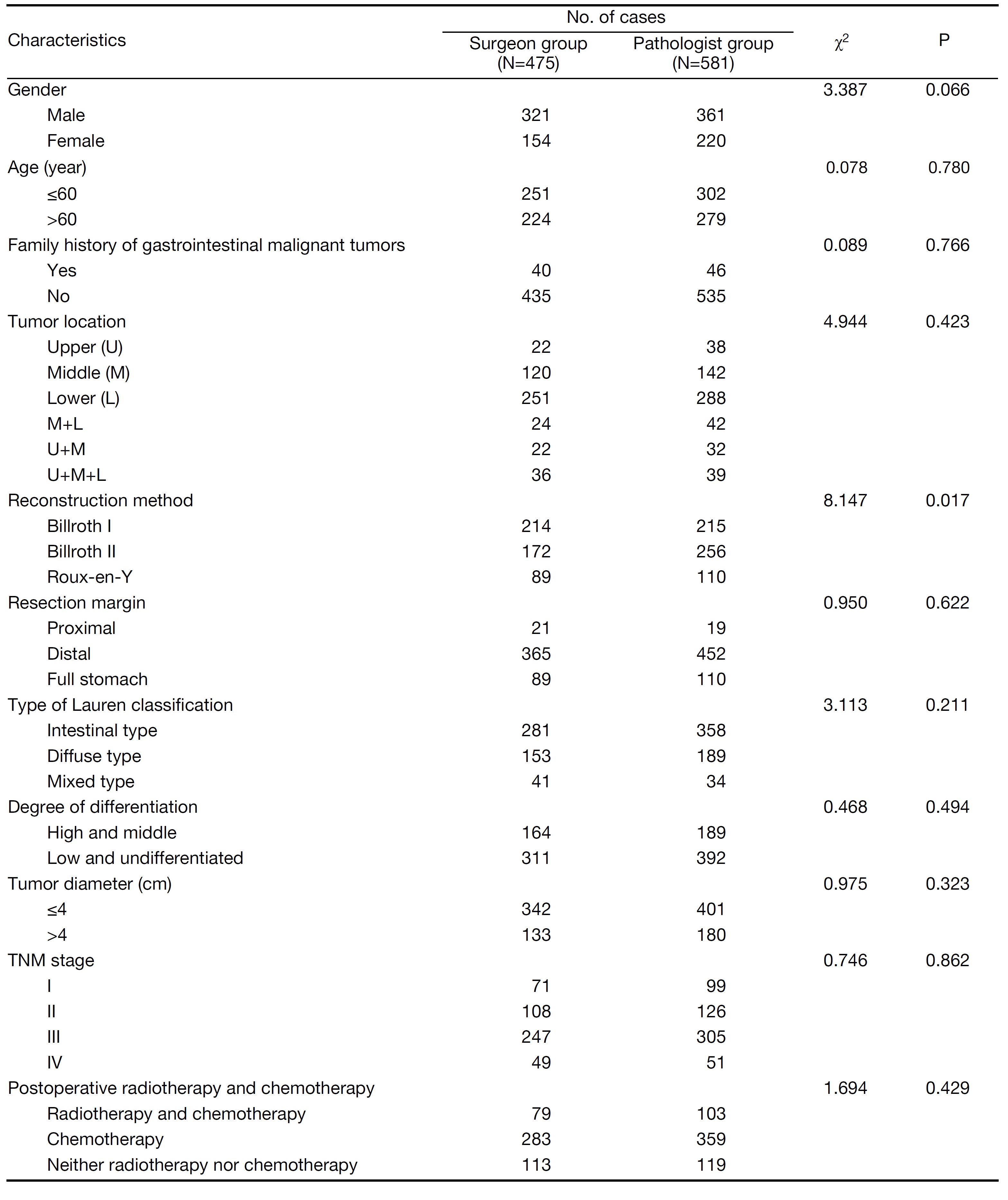
There were no significant differences in gender, age, family history of gastrointestinal malignant tumors, tumor location, resection margin, type of Lauren classification, degree of differentiation, tumor diameter, TNM stage, or the use of postoperative radiotherapy or chemotherapy between the two groups. Only the digestive tract reconstruction method differed between the two groups (P>0.05) (Table 1).
Full table
Number of lymph nodes
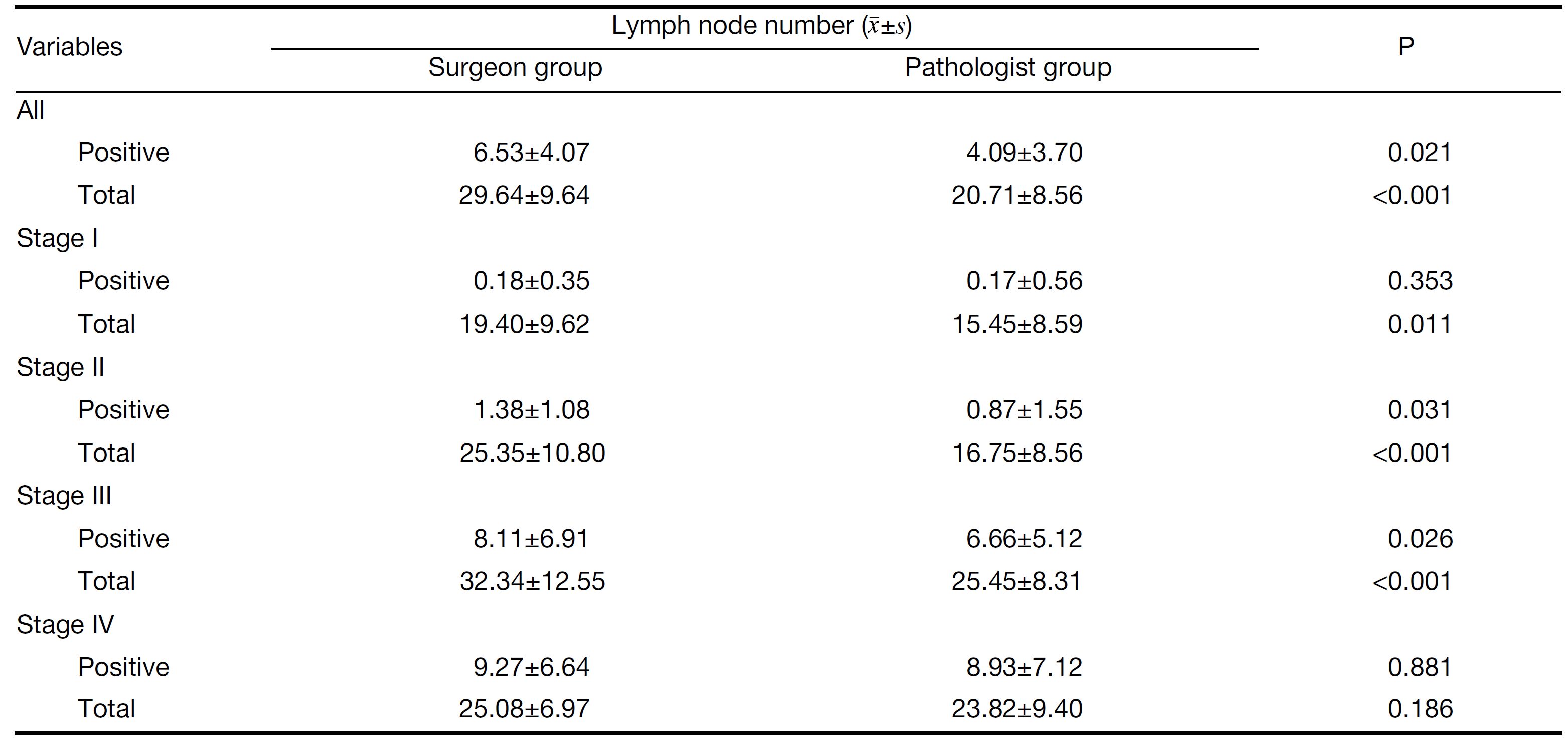
In all, the data of positive and total lymph nodes in each group are in accord with normal distribution. We compared the number of lymph nodes by t-test. As shown in Table 2, the numbers of positive and total lymph nodes were significantly higher in the surgeon group than in the pathologist group (P=0.021, P<0.001, respectively). Further analysis showed that the total number of lymph nodes in stage I patients and the numbers of positive and total lymph nodes in stage II and stage III patients were higher in the surgeon group than in the pathologist group, and these differences were statistically significant (all P<0.05). The number of positive lymph nodes in stage I patients and the numbers of positive and total lymph nodes in stage IV patients were also higher in the surgeon group than in the pathologist group, however, these differences were not statistically significant (all P>0.05).
Full table
Survival analysis
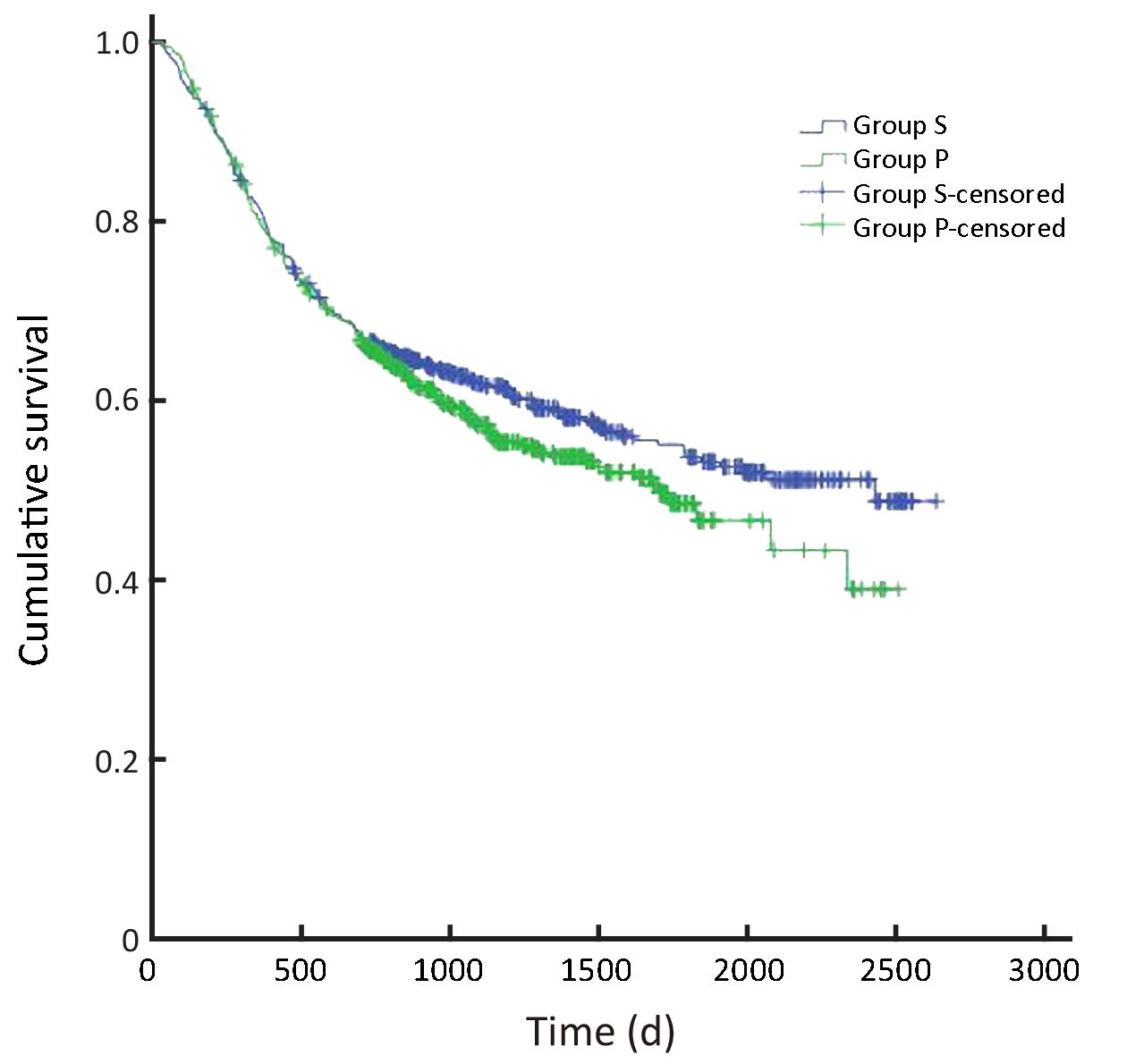
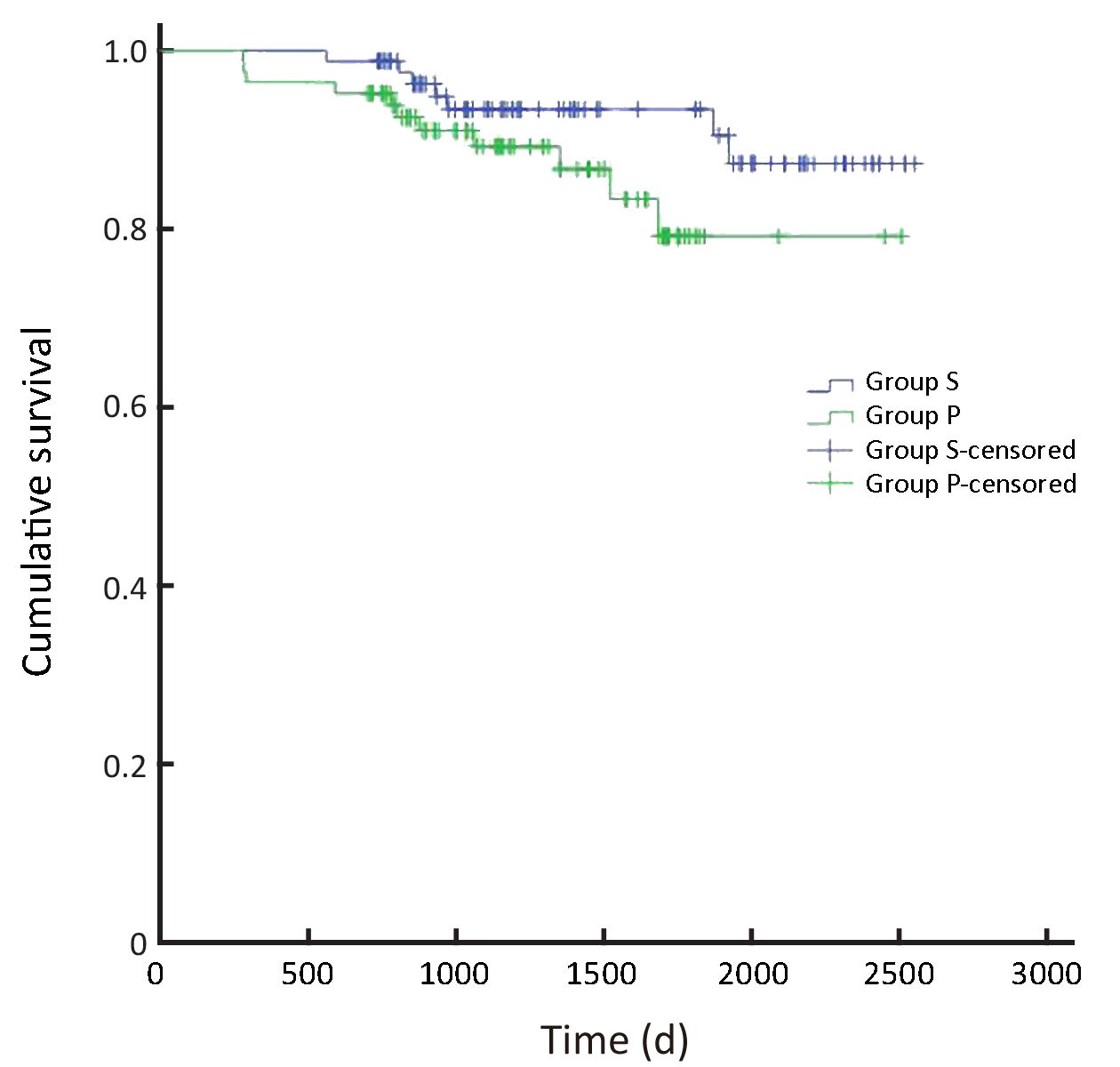
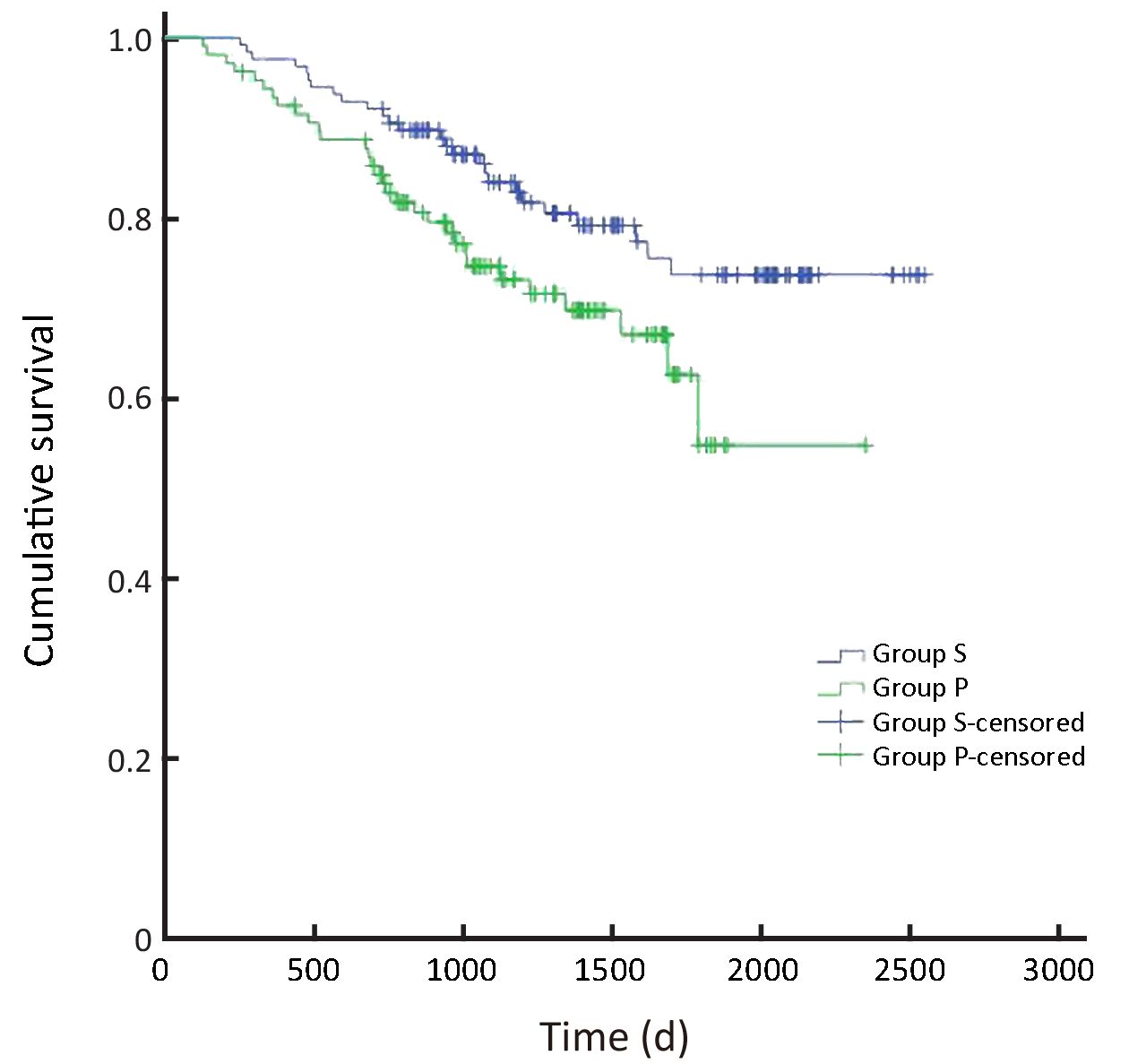
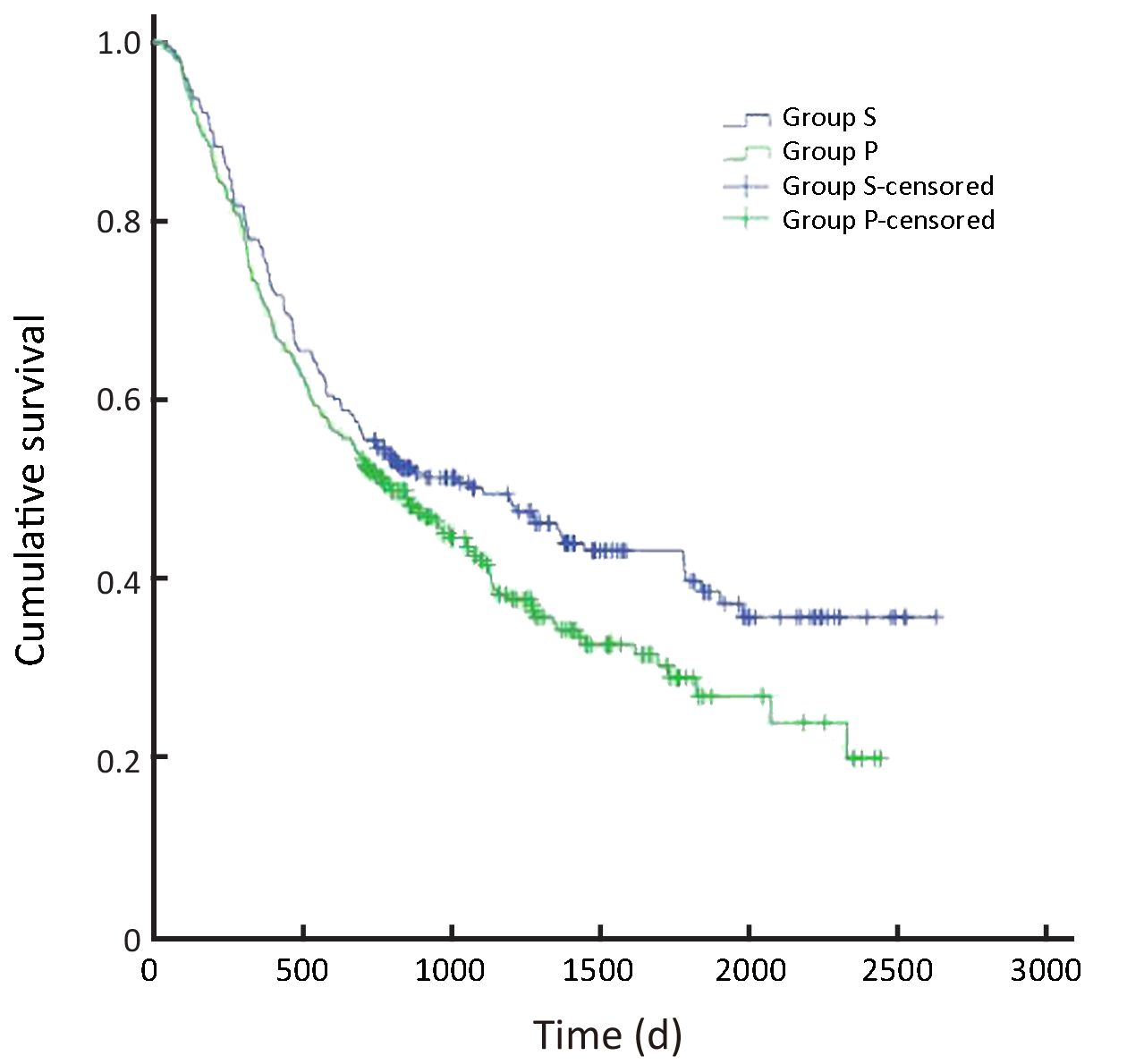
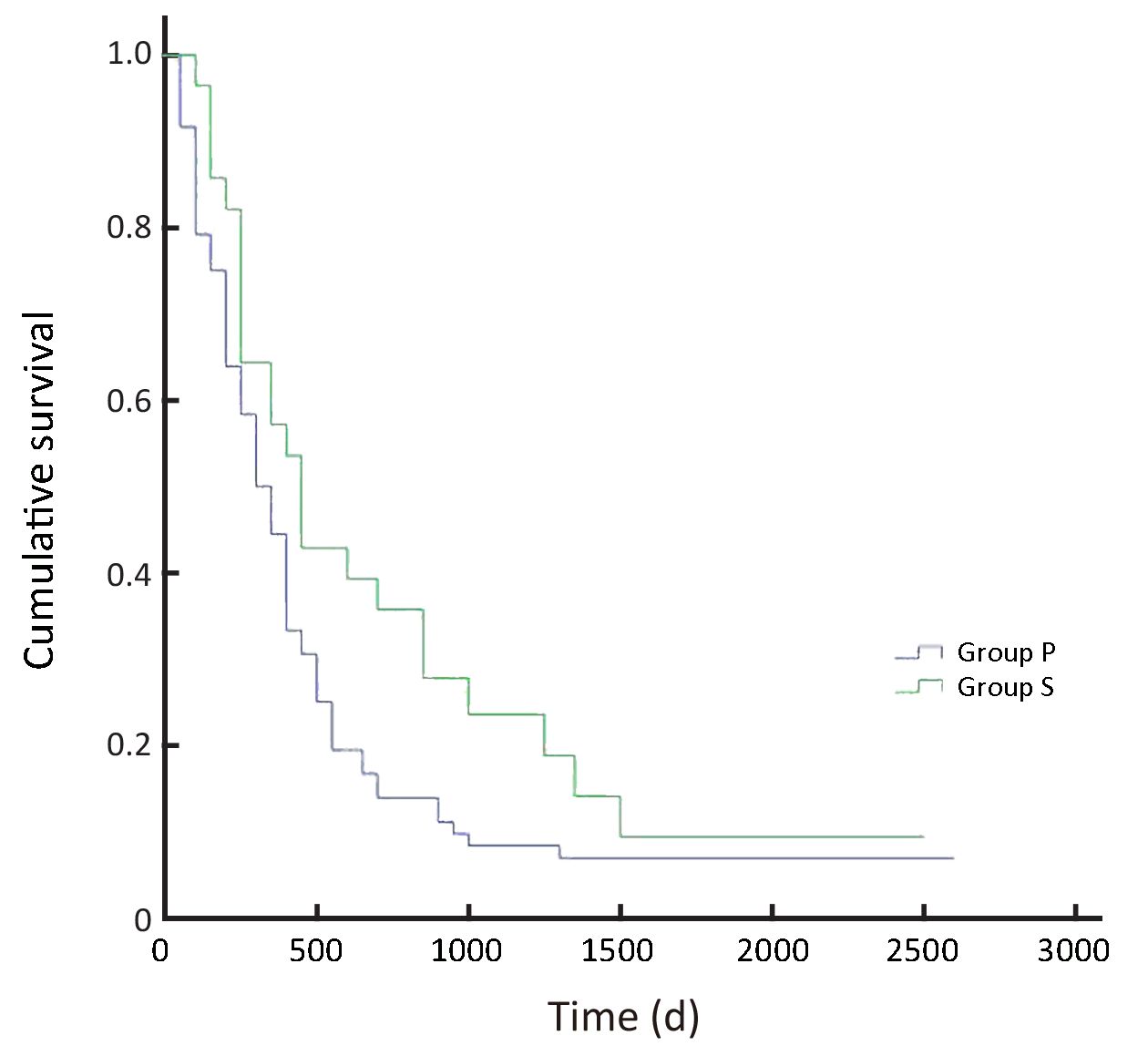
In all, the 3- and 5-year survival rates of patients in the surgeon group were higher than those in the pathologist group, but the difference was not statistically significant (P=0.168). Further analysis showed that the survival rates of stage II and III patients were significantly higher in the surgeon group than in the pathologist group (P=0.038, P=0.045, respectively). Patients with stage I and IV gastric cancer in the surgeon group also had higher survival rates than those in the pathologist group, but these differences were not statistically significant (P=0.224, P=0.268, respectively) (Table 3,Figure 2-6).
Full table
Discussion
In most gastric cancer centres, lymph nodes are retrieved by pathologists (16). However, pathologists always simply report the positive and total numbers of greater and lesser curvature lymph nodes because they are less familiar with gastric lymph node stations. The UICC guidelines recommend that the total number of harvested lymph nodes in gastric cancer patients should be not less than 16, and lymph node retrieval should be considered incomplete if less than 16 lymph nodes are retrieved (17). But it did not recommend the details of perigastric lymph node retrieval. Radiotherapy and chemotherapy have been recommended to prevent recurrence in patients with less than 16 lymph nodes harvested. However, most gastric cancer surgeons think that those cases with less than 16 lymph nodes harvested occur due to insufficient postoperative gastric lymph node retrieval by pathologists rather than their poor surgical skills. En bloc lymph node dissection is often used in patients with gastric cancer (18). It is difficult to determine the number of positive and total lymph nodes in each station if surgeons do not retrieve lymph nodes by station. At present, only the total lymph node number and the positive lymph node number are considered in the National Comprehensive Cancer Network (NCCN) staging guidelines; therefore, pathologists may be satisfied when the number of retrieved perigastric lymph nodes is more than 16. In China, Huang et al. have advised the number of lymph nodes should be more than 21 in radical total gastrectomy to ensure the standard D2 radical lymph node dissection for gastric cancer (19).
However, when lymph node retrieval is performed by surgeons, the surgeons may not only divide lymph nodes into stations but also count sectioned lymph nodes as a single lymph node at each station. This methodological change may greatly reduce the workload of pathologists, increase the total number of perigastric lymph nodes and the number of positive perigastric lymph nodes, improve quality control in the surgical treatment of gastric cancer, and promote the implementation of standard D2 radical lymph node dissection for gastric cancer (20,21). This methodology may also cause the pathological stages of gastric cancer to shift, resulting in patients initially diagnosed with stage II gastric cancer being diagnosed with stage III gastric cancer. In this study, the false negative rate was lower in patients with stage II gastric cancer in the surgeon group; patients shifted from stage II to stage III gastric cancer may accept more radiotherapy and chemotherapy treatment; therefore, this differential diagnosis may improve the survival of patients with stage II gastric cancer. For stage III patients, we hypothesize that the survival may be improved because of improved quality control in the surgical treatment of gastric cancer. Surgeons may improve lymph node dissection by retrieving the lymph nodes, thereby ensuring the implementation of standard D2 radical lymph node dissection. Additionally, the survival of patients in this study was found to be significantly increased, most likely because more positive lymph nodes were harvested.
Conclusions
Compared with retrieval performed by pathologists, postoperative perigastric lymph node retrieval performed by surgeons was associated with significant increase in the total lymph node number of stage I patients, the numbers of positive and total lymph nodes of stage II and III patients, and the survival of stage II and stage III gastric cancer patients. And we suggest that postoperative gastric cancer lymph nodes should be retrieved not only by stations but also by surgeons and then submitted to the Department of Pathology for further examination. This method may be helpful not only in accurately staging patients with gastric cancer and facilitating a decision regarding the subsequent use of radiotherapy and chemotherapy treatment but also in improving the survival of patients with gastric cancer significantly.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [PubMed] DOI:10.3322/caac.21262
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [PubMed] DOI:10.3322/caac.21338
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12. [PubMed]
- Yang L, Yuan Y, Sun T, et al. Population-based cancer incidence analysis in Beijing, 2008-2012. Chin J Cancer Res 2015;27:13–21. [PubMed]
- Akhavan A, Binesh F, Seifaddiny A. Results of combination chemotherapy and radiation therapy in non-metastatic gastric cancer in Yazd--Iran. Indian J Cancer 2015;52:40–3. [PubMed] DOI:10.4103/0019-509X.175583
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745–8. [PubMed] DOI:10.1016/S0140-6736(95)90637-1
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–14. [PubMed] DOI:10.1056/NEJM199903253401202
- Liang Y, Wu L, Wang X, et al. Positive impact of adding No.14v lymph node to D2 dissection on survival for distal gastric cancer patients after surgery with curative intent. Chin J Cancer Res 2015;27:580–7. [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453–62. [PubMed] DOI:10.1056/NEJMoa0707035
- de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg 2013;30:96–103. [PubMed] DOI:10.1159/000350873
- Ji J, Shan F. Pay more attention to the standardized oncologic assessment of gastric cancer prior to starting therapy. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2015;18:104–7. [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430–6. [PubMed] DOI:10.1200/JCO.2009.26.9654
- Kurokawa Y, Sasako M. Recent advances in chemotherapy and chemoradiotherapy for gastrointestinal tract cancers: adjuvant chemoradiotherapy for gastric cancer. Int J Clin Oncol 2008;13:479–82. [PubMed] DOI:10.1007/s10147-008-0848-1
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49. [PubMed] DOI:10.1016/S1470-2045(10)70070-X
- Li Z, Ao S, Bu Z, et al. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: a prospective randomized trial. World J Surg Oncol 2016;14:88. [PubMed] DOI:10.1186/s12957-016-0835-3
- Hu B, El Hajj N, Sittler S, et al. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol 2012;3:251–61. [PubMed]
- Ajani JA, Barthel JS, Bekaii-Saab T, et al. Gastric cancer. J Natl Compr Canc Netw 2010;8:378–409. [PubMed]
- Fu QY, Cui Y, Li XB, et al. Relevant risk factors of positive lateral margin after en bloc endoscopic submucosal dissection for early gastric adenocarcinoma. J Dig Dis 2016;17:244–51. [PubMed] DOI:10.1111/cdd.2016.17.issue-4
- Lu J, Wang W, Zheng CH, et al. Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: an analysis from a two-institution database in China. Ann Surg Oncol 2016 Sep 12. [Epub ahead of print]
- Hu X. Delicate management of gastric cancer surgery process and surgical quality control. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2015;18:111–5.
- Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg 1997;84:1567–71. [PubMed] DOI:10.1002/(ISSN)1365-2168