Dual effect of WISP-1 in diverse pathological processes
Introduction
Wingless-type MMTV integration site family member 1 (Wnt-1)-inducible signaling pathway (WISP) proteins are a subfamily of the cysteine-rich protein 61 (Cyr61)/connective tissue growth factor (CTGF)/nephroblastoma overexpressed (NOV) (CCN) family. Proteins from the CCN family share functional but not sequential similarity to Wnt-1 (1), which belongs to an expanded family of cysteine-rich, glycosylated signaling proteins that mediate diverse developmental processes (2). Members of the CCN family were first identified as secreted proteins whose synthesis were induced by mitogenic growth factors or oncogenes, and were deregulated in transformed cells. The first three members, Cyr61, CTGF and NOV, which are also known as CCN-1, CCN-2 and CCN-3, respectively, contribute to the acronym for the CCN family (3-5). Thereafter three other family members WISP-1, WISP-2 and WISP-3 were designated as CCN-4, CCN-5 and CCN-6, respectively (6). WISP-1 was identified as an indirect response to Wnt-1 stimulation in C57MG mouse mammary epithelial cells (7), whereas WISP-2 was found down-regulated following transformation of rat embryo fibroblasts by the inactivation of p53 and concomitant activation of H-ras (8).
Previous study has reviewed the expression profiles and the WISP-2 related signaling pathways in cancers (9). There are more than 200 publications related to WISP-1, WISP-2, WISP-3 and their roles in cell signaling, proliferation, adhesion, invasion, wound healing, fibrosis, skeletal development, implantation, epithelial-mesenchymal-transition and angiogenesis, as well as in cancers. Here in this review, we provided with a brief introduction on the expression profiles, clinical significance, functions and potential mechanisms of WISP-1 in cancer and non-neoplastic diseases.
Chromosome location, structure and variants of WISP-1
WISP-1 gene is located on chromosome 15 in murine, but on 8q24.1-q24.3 in human. Both of them are composed of five exons and four introns, and the cDNA length is 1,766 bp and 2,830 bp, respectively. The human WISP-1 encodes 367 amino acids (84% identical to the murine one), including 38 conserved cysteine residues and four potential N-linked glycosylation sites. In addition, the WISP-1 gene encoded protein has a predicted relative molecular mass of 40 kD (1,10).
An alignment of the three human WISP proteins showed that WISP-1 and WISP-3 are most similar (42% identity), whereas WISP-2 is 37% identical to WISP-1. WISP proteins exhibit the modular architecture of CCN family members that are characterized as four conserved and discrete cysteine-rich domains. These domains act both independently and in concert. The N-terminal domain, including the first twelve cysteine residues, contains a consensus sequence of GCGCCXXC which is conserved in most insulin-like growth factor-binding proteins (IGFBPs). The von Willebrand factor type C (VWC) module covers the next ten cysteine residues, and is thought to participate in protein complex formation and oligomerization (11). A short variable region following closely to the VWC domain is highly susceptible to proteolytic degradation by matrix metalloproteinases (MMPs) (12). It has been shown that a wide variety of MMPs (MMP1, 2, 3, 7, 9, 13) target this central linker region and additional proteases, such as elastase, additionally, plasmin could also attack linkers that connect domains 1 and 2 or domains 3 and 4 (13). The third domain, thrombospondin (TSP) domain is implicated in binding with sulfatedglyco conjugates and contains six cysteine residues and a conserved WSxCSxxCG motif, which was first identified in TSP (14) and necessary for the regulation of endothelial cell proliferation and the promotion of cell attachment (15,16). The C-terminal cysteine knot-like (CT) domain which has been indicated in all CCN family members except for WISP-2 was implicated in receptor binding and dimerization (17).
CCN family members have a topical structure that is consist of four conserved cysteine-rich modular domains, and it is indicated that lack of one or more modules would result in variants splicing or gene mutation. Three variants have been discovered in addition to the expected full transcript length of WISP-1/CCN4 (1,204 bp). One of the variants, WISP-1v that is short of the VWC module II, was detected in scirrhous gastric carcinoma cells. This variant is encoded by alternatively spliced 840 nucleotides mRNA species, missing the 260 nucleotides exon3 that encodes VWC module (18). The second variant, WISP-1vx, lacks VWC, TSP and partial IGFBP domains, and the IGFBP domain is shorter than its full length, which was caused by a frame shift leading to IGFBP/CT fusion. The protein contains a single IGFBP module, in which eight C-terminal amino acid residues are replaced by another fourteen residues, and such variant was detected in two hepatocellular carcinoma cell lines (19). The third variant, WISP-1 Eex3−4, contains a splice variation, leading to a shorter transcript length of 750 nucleotides, and has the frame shift of exons 2 and 5 that results in a premature stop. The predicted protein of this variant has only the first module, and this variant was detected in a human chondrosarcoma-derived chondrocytic cell line (20).
WISP-1 expression and clinical significance in human cancers
Differential expression and prognostic implications of WISP-1, WISP-2 and WISP-3 were detected in human colorectal (21) and breast (22) cancers. Davies et al . found that WISPs may play important but contrasting roles. In colorectal cancer, WISP-1 appeared to act as a factor stimulating aggressiveness, however, WISP-1 seemed to act as a tumor suppressor in breast cancer. Notably, the expression profile of WISP-2 in colorectal cancer and breast cancer tissues demonstrated contrasting results to that of WISP-1, indicating the distinct role of WISP-1 and WISP-2 in caner progression and metastasis. Interestingly, it seems that WISP-3 has no definable beneficial or detrimental role in accordance with the expression profile in colorectal and breast cancer tissues. The contrasting roles of WISP-1 in colorectal and breast cancer hinted us that their roles may be tissue specific. The members of CCN family, Cyr61 and CTGF, were down-regulated, but WISP-1 was overexpressed in lung cancer. Moreover, tumor type, age and family history were suggested to be significant predictors for WISP-1 expression, and Cyr61 and CTGF were correlated with survival of lung cancer patients. The Cyr61, CTGF and WISP-1 could play important roles in the development of lung cancer (23,24). WISP-1 which was up-regulated in esophageal squamous cell carcinoma (ESCC) was found to mediate radioresistance through inhibiting irradiation-induced DNA damage, and acted as a prognosis factor in ESCC (25). The expression of WISP-1 was higher in hepatocellular carcinoma (HCC) than in matched normal tissues, and hepatitis B virus (HBV) X protein (HBX) could increase the expression level of WISP-1. Taken together, HBX may play roles in the expression of WISP-1 and the development of HCC (26). As a variant of WISP-1, WISP-1v was overexpressed in cholangiocarcinoma, and had strong correlation with lymphatic and perineural invasion of tumor cells. WISP-1v was involved in the development of cholangiocarcinoma and the generation of invasive cellular properties of tumor cells (27). WISP-1v was also reported to be overexpressed in scirrhous gastric carcinoma, whereas it promoted significant cellular transformation and rapid growth, and enhanced the invasive phenotype of co-cultured gastric carcinoma cells (18). Moreover, in human glioma, cells with high expression of WISP-1 had low radiosensitive but high migration activity in an in vitro study (28). Another in vitro study on colon cancer cell line demonstrated that both transcript and protein expression levels of WISP-1 were down-regulated following the treatment of indomethacin, an anti-inflammatory drug that inhibited tumor growth, through a cyclooxgenase-2 (COX-2)-independent manner, emphasizing the potential regulatory role of WIPS-1 in cancer cell proliferation (29).
Signaling regulation of WISP-1 in cancers
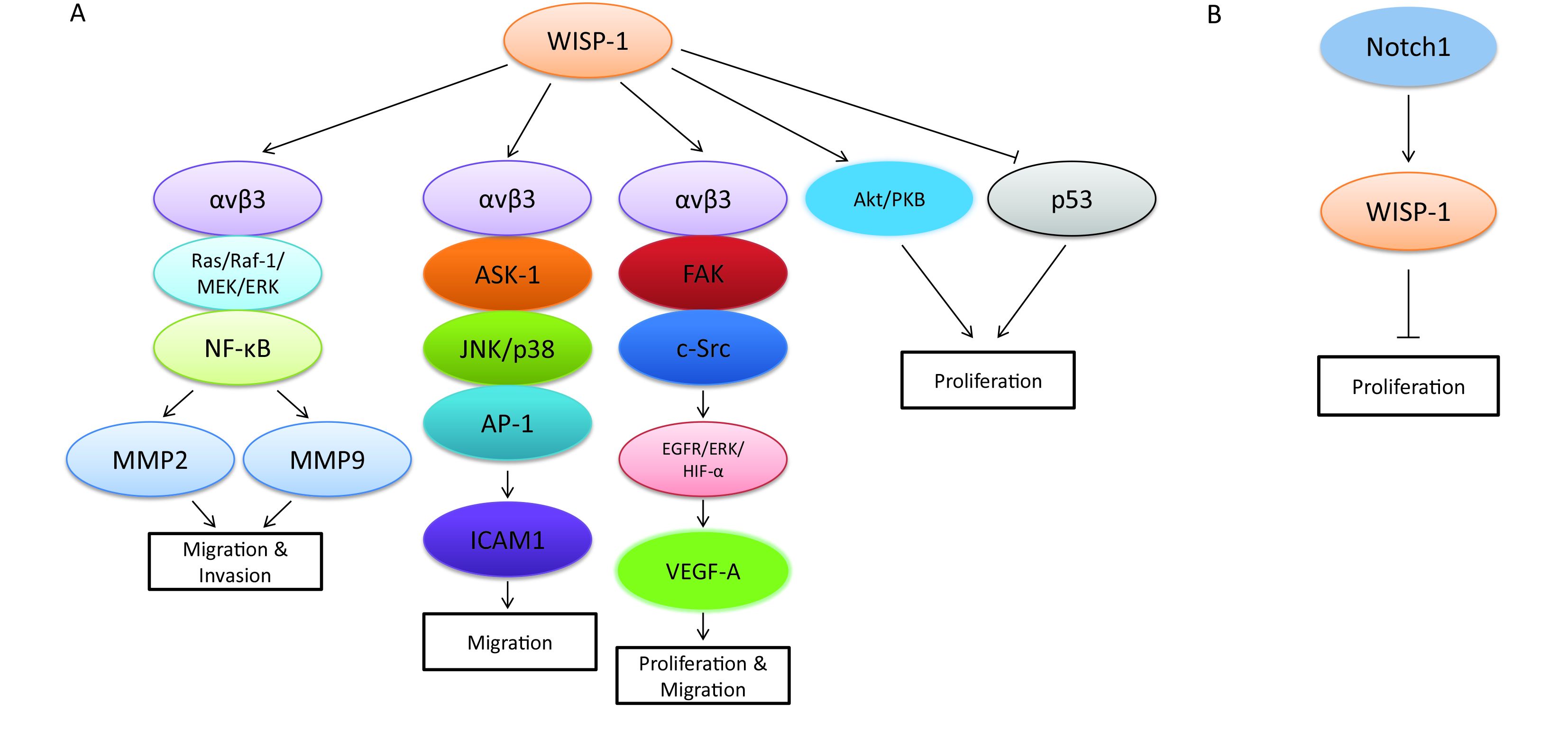
Although WISP-1 itself is a downstream target gene of Wnt/β-catenin signaling pathway, it also plays an essential role in regulating the complicated network which is composed of various signaling transduction pathways and associated with the progression of different biological process in diverse tumor types. Recent research revealed that WISP-1 functioned as a novel angiogenic regulator which stimulated growth through the induction of vascular endothelial growth factor A (VEGF-A), which was identified as an important angiogenic factor involved in tumor development in oral squamous cell carcinoma (OSCC). The special molecular mechanism mediated by WISP-1 in this biological process was via triggering integrin αvβ3/FAK/c-Src pathway, which further transactivated the epidermal growth factor receptor (EGFR)/signal-regulated kinase (ERK)/hypoxia-inducible factor 1-alpha (HIF1-α) signal pathway in OSCC (30). Besides, WISP-1 was also demonstrated to be involved in promoting OSCC cell migration through the activation of αvβ3 integrin receptor and the signaling transduction pathways of apoptosis signal regulating kinase-1 (ASK-1), JNK/p38, and activator protein-1 (AP-1), which then up-regulated the expression of intercellular adhesion molecule 1 (ICAM1) (31). Similar biological phenomenon was found in human osteosarcoma, whereas the migration process mediated by WISP-1 was mainly through the activation of MMP2 and MMP9 via αvβ3 integrin receptor and the signaling pathways of Ras/Raf-1/MEK/ERK and NF-κB, which differed from the molecular mechanism in OSCC to a certain degree (32). In addition, WISP-1 promoted the motility and metastasis through the activation of Ras pathway and the enhancement of MMP2 (33). However, other studies demonstrated that WISP-1 potentially inhibited cancer metastasis in lung cancer and prostate cancer (34,35). As a variant of WISP-1, WISP-1v was overexpressed in cholangiocarcinoma and was highly correlated with lymphatic and perineural invasion of tumor cells through the mitogen-activated protein kinases (MAPKs) p38 and p42/p44 (27). Moreover, another way to accelerate cell growth mediated by WISP-1 was via “anti-apoptosis”. WISP-1 was confirmed to not only activate the antiapoptotic protein kinase B (PKB)/Akt signaling pathway, but also prevent p53-dependent cell death in lung carcinoma cells and breast cancer cells (36). Despite of the oncogenic role in diverse tumor types, WISP-1 was also found to act as a tumor suppressor via distinct mechanisms in several tumor types in recent studies. Notch1 was one of the main upstream molecular signaling pathways that involved in this process, which could further magnify the biological progression such as cell proliferation, migration and metastasis (37,38). The signaling pathways that WISP-1 participated in are summarized in Figure 1.
WISP-1 proteins in other pathologic processes
Apart from the effects of WISP-1 on cancer formation and progression, WISP-1 was also found to participate in a variety of other pathological processes. A study showed that WISP-1 could potentially be involved in the regulation of adipogenesis and low-grade inflammation in obesity due to the fact that the expression of WISP-1 was found associated with the differentiation of adipocyte and that pro-inflammatory response was found in the WISP-1 stimulated human macrophages (39). WISP-1 was highly expressed in many fibrosis diseases, such as liver fibrosis and idiopathic pulmonary fibrosis (IPF). In liver fibrosis, WISP-1 promoted the proliferation of hepatic stellate cells (HSCs) (40), whereas the expression of WISP-1 was regulated by miR92a at regions of human WISP-1 3’-untranslated region (3’UTR) in IPF (41). WISP-1 was also involved in the regulation of osteogenesis through the association with integrin, which could mediate osteogenesis effects of bone morphogenetic protein-2 (BMP-2) (42). Another study demonstrated that myocardial hypertrophy was induced by Angiotensin-II (Ang-II) in a WISP-1-dependent manner, suggesting the important role of WISP-1 in myocardial hypertrophy (43). In addition, the polymorphisms of WISP-1 were significantly correlated with hypertension of Japanese male (44). WISP-1 can enhance the neuronal cells survival through the suppression of Bim/Bax complex and the promotion of Bclx(L)/Bax complex, respectively, preventing mitochondrial membrane permeability, cytochrome c release, and caspase 3 activation in the presence of oxidant stress through phosphoinositide 3-kinase (PI3K) and Akt pathways (45).
Mechanisms of WISP-1 in other pathologic processes
Proliferation
WISP-1 belongs to the members of CCN family of growth factors and plays important roles in cell proliferation. In post-infarct heart, pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) activated cAMP response element-binding protein (CREB) through ERK1/2 to increase the expression of WISP-1, which in turn promoted myocardial cell proliferation and remodeling (46). A study on human bone marrow stroma cells (hBMSCs) suggested that WISP-1 modulated the proliferation and differentiation of osteoblast-like cell potentially via the reduction of the phosphorylation of TGF-1 induced Smad-2 (47).
Anti-apoptosis
WISP-1 was also found involved in anti-apoptosis of neuronal cell through PI3K, Akt, β-catenin and mammalian target of rapamycin (mTOR). In accompany with PI3K and Akt, WISP-1 improved post-translation phosphorylation of forkhead transcription factor 3a (FoxO3a) and sequestered FoxO3a to prevent caspase-3 mediated apoptotic cell death in primary neurons under oxidative stress (48).
Adhesion and migration
WISP-1 is a matricellular protein of CCN family. In osteoarthritis synovial fibroblasts (OASFs), WISP-1 promoted the expression of vascular cell adhesion molecule-1 (VCAM-1) to increase the adhesion of monocytesvia the PKC-delta, JNK, c-Jun and AP-1 signaling pathways (49). In cultured rat vascular smooth muscle cells (VSMCs), WISP-1 mediated the adhesion of VSMCs, which could be reversed by an antibody against integrin. Moreover, WISP-1 was also found to stimulate the migration of VSMCs via integrin (50).
Conclusions
WISP-1 is a member of CCN family of growth factors with appealing roles in multiple tissues and pathologic approaches, including cancer, fibrosis, myocardial hypertrophy and hypertension. WISP-1 generally behaves as an oncogene that is highly expressed in different cancers and correlated with the progression, distant metastasis and poor prognosis of cancer patients; however it was also found to be a tumor suppressor in certain tissue types. WISP-1 can stimulate proliferation, adhesion, invasion, metastasis and epithelial-to-mesenchymal transition of cancer cells. Being one of the β-catenin regulated factors in the Wnt/β-catenin signaling pathway, WISP-1 holds the potential role to act as a novel therapeutic target for cancer therapy.
Acknowledgements
Funding: This article was supported by the Young Talents of Science and Technology Support Project of Colleges and Universities of Inner Mongolia Autonomous Region (NJYT-12-B21, 2012), the Great Project of the Affiliated Hospital of Inner Mongolia Medical University, China, 2012 (No. NYFY ZD 2012014), and Beijing Municipal Administration of Hospitals’ Youth Programme (QML20151003).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pennica D, Swanson TA, Welsh JW, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A 1998;95:14717–22. [PubMed] DOI:10.1073/pnas.95.25.14717
- Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 2006;39:5–16. [PubMed] DOI:10.1016/j.bone.2005.12.008
- O’Brien TP, Yang GP, Sanders L, et al. Expression of cyr 61, a growth factor-inducible immediate-early gene. Mol Cell Biol 1990;10:3569–77. [PubMed] DOI:10.1128/MCB.10.7.3569
- Bradham DM, Igarashi A, Potter RL, et al. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 1991;114:1285–94. [PubMed] DOI:10.1083/jcb.114.6.1285
- Joliot V, Martinerie C, Dambrine G, et al. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 1992;12:10–21. [PubMed] DOI:10.1128/MCB.12.1.10
- Perbal B, Brigstock DR, Lau LF. Report on the second international workshop on the CCN family of genes. Mol Pathol 2003;56:80–5. [PubMed] DOI:10.1136/mp.56.2.80
- Mason JO, Kitajewski J, Varmus HE. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol Biol Cell 1992;3:521–33. [PubMed] DOI:10.1091/mbc.3.5.521
- Zhang R, Averboukh L, Zhu W, et al. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol 1998;18:6131–41. [PubMed] DOI:10.1128/MCB.18.10.6131
- Ji J, Jia S, Ji K, et al. Wnt1 inducible signalling pathway protein-2(WISP-2/CCN5): roles and regulation in human cancers (review). Oncol Rep 2014;31:533–9. [PubMed] DOI:10.3892/or.2013.2909
- Berschneider B, Königshoff M. WNT1 inducible signaling pathway protein 1(WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol 2011;43:306–9. [PubMed] DOI:10.1016/j.biocel.2010.11.013
- Mancuso DJ, Tuley EA, Westfield LA, et al. Structure of the gene for human von Willebrand factor. J Biol Chem 1989;264:19514–27. [PubMed]
- Hashimoto G, Inoki I, Fujii Y, et al. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 2002;277:36288–95. [PubMed] DOI:10.1074/jbc.M201674200
- de Winter P, Leoni P, Abraham D. Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein. Growth Factors 2008;26:80–91. [PubMed] DOI:10.1080/08977190802025602
- Holt GD, Pangburn MK, Ginsburg V. Properdin binds to sulfatide [Gal(3-SO4)beta 1-1 Cer] and has a sequence homology with other proteins that bind sulfated glycoconjugates. J Biol Chem 1990;265:2852–5. [PubMed]
- Karagiannis ED, Popel AS. Peptides derived from type I thrombospondin repeat-containing proteins of the CCN family inhibit proliferation and migration of endothelial cells. Int J Biochem Cell Biol 2007;39:2314–23. [PubMed] DOI:10.1016/j.biocel.2007.06.018
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 1993;327:125–30. [PubMed] DOI:10.1016/0014-5793(93)80155-N
- Voorberg J, Fontijn R, Calafat J, et al. Assembly and routing of von Willebrand factor variants: the requirements for disulfide-linked dimerization reside within the carboxy-terminal 151 amino acids. J Cell Biol 1991;113:195–205. [PubMed] DOI:10.1083/jcb.113.1.195
- Tanaka S, Sugimachi K, Saeki H, et al. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene 2001;20:5525–32. [PubMed] DOI:10.1038/sj.onc.1204723
- Yanagita T, Kubota S, Kawaki H, et al. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J 2007;274:1655–65. [PubMed] DOI:10.1111/j.1742-4658.2007.05709.x
- Cervello M, Giannitrapani L, Labbozzetta M, et al. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann N Y Acad Sci 2004;1028:432–9. [PubMed] DOI:10.1196/annals.1322.051
- Davies SR, Watkins G, Mansel RE, et al. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann Surg Oncol 2007;14:1909–18. [PubMed] DOI:10.1245/s10434-007-9376-x
- Davies SR, Davies ML, Sanders A, et al. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol 2010;36:1129–36. [PubMed] DOI:10.3892/ijo_00000595
- Li N, Wang Q, Chen P, et al. Study on expression of CTGF and WISP-1 genes in human lung cancers. Wei Sheng Yan Jiu (in Chinese) 2008;37:555–7. [PubMed]
- Chen PP, Li WJ, Wang Y, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One 2007;2:e534. [PubMed] DOI:10.1371/journal.pone.0000534
- Zhang H, Luo H, Hu Z, et al. Targeting WISP1 to sensitize esophageal squamous cell carcinoma to irradiation. Oncotarget 2015;6:6218–34. [PubMed] DOI:10.18632/oncotarget
- Jiang X, Wang ZJ, Xie QH, et al. WISP-1: a novel mediator of hepatitis B virus-related hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi (in Chinese) 2013;21:285–9. [PubMed] DOI:10.3760/cma.j.issn.1007-3418.2013.04.011
- Tanaka S, Sugimachi K, Kameyama T, et al. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology 2003;37:1122–9. [PubMed] DOI:10.1053/jhep.2003.50187
- Yu W, Song T, Shi L, et al. Targeted inhibition of WISP1 enhanced radiosensitivity in glioma cells. Zhonghua Yi Xue Za Zhi (in Chinese) 2014;94:1507–11. [PubMed] DOI:10.3760/cma.j.issn.0376-2491.2014.19.019
- Wang J, Zhang GY, Li XH. Effect of indomethacin on Bfl-1, WISP-1 and proliferating cell nuclear antigen in colon cancer cell line HCT116 cells. Chin J Dig Dis 2006;7:219–24. [PubMed] DOI:10.1111/cdd.2006.7.issue-4
- Chuang JY, Chen PC, Tsao CW, et al. WISP-1 a novel angiogenic regulator of the CCN family promotes oral squamous cell carcinoma angiogenesis through VEGF-A expression. Oncotarget 2015;6:4239–52. [PubMed] DOI:10.18632/oncotarget
- Chuang JY, Chang AC, Chiang IP, et al. Apoptosis signal-regulating kinase 1 is involved in WISP-1-promoted cell motility in human oral squamous cell carcinoma cells. PLoS One 2013;8:e78022. [PubMed] DOI:10.1371/journal.pone.0078022
- Wu CL, Tsai HC, Chen ZW, et al. Ras activation mediates WISP-1-induced increases in cell motility and matrix metalloproteinase expression in human osteosarcoma. Cell Signal 2013;25:2812–22. [PubMed] DOI:10.1016/j.cellsig.2013.09.005
- Hou CH, Chiang YC, Fong YC, et al. WISP-1 increases MMP-2 expression and cell motility in human chondrosarcoma cells. Biochem Pharmacol 2011;81:1286–95. [PubMed] DOI:10.1016/j.bcp.2011.03.016
- Soon LL, Yie TA, Shvarts A, et al. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem 2003;278:11465–70. [PubMed] DOI:10.1074/jbc.M210945200
- Ono M, Inkson CA, Sonn R, et al. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One 2013;8:e71709. [PubMed] DOI:10.1371/journal.pone.0071709
- Su F, Overholtzer M, Besser D, et al. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev 2002;16:46–57. [PubMed] DOI:10.1101/gad.942902
- Liu ZJ, Li Y, Tan Y, et al. Inhibition of fibroblast growth by Notch1 signaling is mediated by induction of Wnt11-dependent WISP-1. PLoS One 2012;7:e38811. [PubMed] DOI:10.1371/journal.pone.0038811
- Shao H, Cai L, Grichnik JM, et al. Activation of Notch1 signaling in stromal fibroblasts inhibits melanoma growth by upregulating WISP-1. Oncogene 2011;30:4316–26. [PubMed] DOI:10.1038/onc.2011.142
- Murahovschi V, Pivovarova O, IIkavets I, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes 2015;64:856–66. [PubMed] DOI:10.2337/db14-0444
- Jian YC, Wang JJ, Dong S, et al. Wnt-induced secreted protein 1/CCN4 in liver fibrosis both in vitro and in vivo. Clin Lab 2014;60:29–35. [PubMed]
- Berschneider B, Ellwanger DC, Baarsma HA, et al. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int J Biochem Cell Biol 2014;53:432–41. [PubMed] DOI:10.1016/j.biocel.2014.06.011
- Ono M, Inkson CA, Kilts TM, et al. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res 2011;26:193–208. [PubMed] DOI:10.1002/jbmr.205
- Shanmugam P, Valente AJ, Prabhu SD, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol 2011;50:928–38. [PubMed] DOI:10.1016/j.yjmcc.2011.02.012
- Yamada Y, Ando F, Shimokata H. Association of polymorphisms of SORBS1, GCK and WISP1 with hypertension in community-dwelling Japanese individuals. Hypertens Res 2009;32:325–31. [PubMed] DOI:10.1038/hr.2009.23
- Wang S, Chong ZZ, Shang YC, et al. Wnt1 inducible signaling pathway protein 1(WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res 2012;9:20–31. [PubMed] DOI:10.2174/156720212799297137
- Venkatachalam K, Venkatesan B, Valente AJ, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem 2009;284:14414–27. [PubMed] DOI:10.1074/jbc.M809757200
- Inkson CA, Ono M, Kuznetsov SA, et al. TGF-beta1 and WISP-1/CCN-4 can regulate each other’s activity to cooperatively control osteoblast function. J Cell Biochem 2008;104:1865–78. [PubMed] DOI:10.1002/jcb.v104:5
- Wang S, Chong ZZ, Shang YC, et al. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res 2013;10:54–69. [PubMed] DOI:10.2174/156720213804805945
- Hou CH, Tang CH, Hsu CJ, et al. CCN4 induces IL-6 production through αvβ5 receptor, PI3K, Akt, and NF-κB singling pathway in human synovial fibroblasts. Arthritis Res Ther 2013;15:R19. [PubMed] DOI:10.1186/ar4151
- Liu H, Dong W, Lin Z, et al. CCN4 regulates vascular smooth muscle cell migration and proliferation. Mol Cells 2013;36:112–8. [PubMed] DOI:10.1007/s10059-013-0012-2
