Non-anthracycline-containing docetaxel and cyclophosphamide regimen is associated with sustained worse outcome compared with docetaxel, anthracycline and cyclophosphamide in neoadjuvant treatment of triple negative and HER2-positive breast cancer patients: updated follow-up data from NATT study
Introduction
Adjuvant systemic chemotherapy has significantly improved the prognosis of breast cancer patients. For patients with locally advanced breast cancer, neoadjuvant chemotherapy is considered as the standard therapy that can improve surgical options (1). Breast cancer patients who achieved pathological complete remission (pCR) after neoadjuvant therapy had a superior outcome compared with those without pCR, especially in triple-negative breast cancer (TNBC) or human epidermal growth factor receptor-2 (HER2)-positive breast cancer. This may offer the opportunity to investigate the chemosensitivity of the potential novel treatment in an in vivo test (2,3).
Anthracycline-and taxane-based regimens are recom- mended as the standard neoadjuvant chemotherapy in breast cancer patients (4). In adjuvant chemotherapy setting, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has displayed that adding taxane to anthracyclines can further reduce the disease recurrence and death in comparison with the anthracycline-based regimens (5). However, several studies, including U.S. Oncology (USO) 9735 trial (6), a meta-analysis (7), and the MA.21 trial subgroup analysis (8), demonstrated that anthracycline-containing regimens were not superior to non-anthracycline-containing regimens in HER2-negative breast cancer patients. Moreover, non-anthracycline-containing docetaxel, carboplatin and trastuzumab (TCH) regimen showed similar efficacy compared with the doxorubicin plus cyclophosphamide followed by docetaxel plus trastuzumab (ACTH) regimen in HER2-positive early breast cancer patients (9). In addition, the usage of anthracyclines was associated with the irreversible cardiac toxicity and severe gastrointestinal side effects (10), which challenged the role of anthracyclines in systemic chemo- therapy of breast cancer.
Our previously published phase III randomized trial (NATT) showed that the pCR rate was numerically higher in TNBC or HER2-positive breast cancer patients treated with anthracycline-containing docetaxel, anthracycline and cyclophosphamide (TAC) than docetaxel plus cyclo-phosphamide (TC) regimen (11). With a median follow-up period of 20 months, TAC treatment was associated with a superior event-free survival (EFS) outcome than the TC treatment, especially in TNBC patients, which leads to a premature cessation of recruitment for the trial studies. The joint analysis of Anthracyclines in Breast Cancer (ABC) trials also failed to demonstrate the non-inferiority of non-anthracycline-containing TC regimen compared with taxanes and doxorubicin plus cyclophosphamide (AC) regimens in HER2-negative patients (12).
To further validate our previous follow-up findings, we continued to follow up the patients who had been enrolled in and completed the neoadjuvant treatment. Here, we reported our updated long-term follow-up data about the NATT study to examine the role of anthracycline in neoadjuvant treatment of TNBC and HER2-positive breast cancer patients.
Materials and methods
Patients
The NATT study was carried out between May 2009 and December 2011, as described previously (11). Briefly, NATT trial was a multicenter, open-label, randomized, non-inferiority, and phase III study in women with TNBC or HER2-positive breast cancer. Estrogen receptor (ER)- or progesterone receptor (PR)-positive was defined as not less than 1% tumor cells with positive cell nuclear staining. HER2-positive breast cancer was defined as HER2 3+ by immunohistochemistry (IHC) or amplification by fluorescence in situ hybridization (FISH). TNBC was defined as ER, PR and HER2 negativity. Patients with stage IIB or III disease according to the American Joint Committee on Cancer (AJCC) staging system (version 6) were eligible for participation in the present study. All patients provided written informed consent before randomization. The Ethical Committee/Institutional Review Board reviewed and approved the protocol that was conducted in accordance with the Declaration of Helsinki and supervised by an Independent Data Monitoring Committee (IDMC).
Procedure and treatment
Patients were randomized to receive either 6 cycles of docetaxel 75 mg/m2 plus cyclophosphamide 600 mg/m2 (TC) on day 1 every 21 days or 6 cycles of docetaxel 75 mg/m2, anthracycline, and cyclophosphamide 500 mg/m2 (TAC) on day 1 every 21 days. Either epirubicin 60 mg/m2 or doxorubicin 50 mg/m2 was considered as an anthra-cycline drug in this study. After surgery, any further chemotherapy was not administered, and all patients were recommended to receive adjuvant radiotherapy. For HER2-positive breast cancer patients, adjuvant trastu-zumab treatment was not mandatory due to its cost and being not covered by insurance at that time. ER- and/or PR-positive patients were treated with anti-estrogen regimens according to the discretion of the treating physician.
Response and outcome
The primary endpoint pCR rate is defined as the absence of invasive tumor in the breast and axillary lymph nodes samples. The secondary endpoints include clinical response, EFS, and overall survival (OS). EFS was calculated as the disease interval between breast cancer diagnosis and the documented disease progression, local and regional recurrences, distant metastasis, secondary malignant carcinoma, or death from any cause. OS was measured from the date of initial diagnosis to the date of the last follow-up or death. Patients were followed up at 3-month intervals during the initial 2 years post-surgery and then at 6-month intervals for the next 3 years.
Statistical analysis
NATT was a phase III, non-inferiority study, designed to compare the efficacy of TC with TAC regimen in the neoadjuvant treatment of TNBC or HER2-positive breast cancer. The 96 enrolled patients were subjected to the Bayesian predictive probability method, suggested by IDMC, to test the possibility of inferior prognosis in TC arm due to its relatively high disease progression or recurrence. This outcome leads to the early stop of enrollments in the study on May 31, 2012, with a median follow-up period of 20 months. The details of the study design modification and statistical considerations were described in our previous report. In the present study, a longer period of follow-up was achieved to calculate the prognosis difference. Kaplan-Meier method was used to calculate the relationship between survival data and the potential influencing factors, such as age, menopausal status, body mass index (BMI), clinical tumor size stage, clinical lymph node stage, ER status, PR status, molecular subtype status, treatment regimens, and pCR status. Next, the factors associated with disease prognosis by log-rank tests were included to calculate the hazard ratio (HR) using the Cox proportional hazards model. HR was presented with their 95% confidence intervals (95% CI). All statistical analyses were performed by IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA) with a two-sided test at a 5% level of significance.
Results
Patient characteristics and treatment
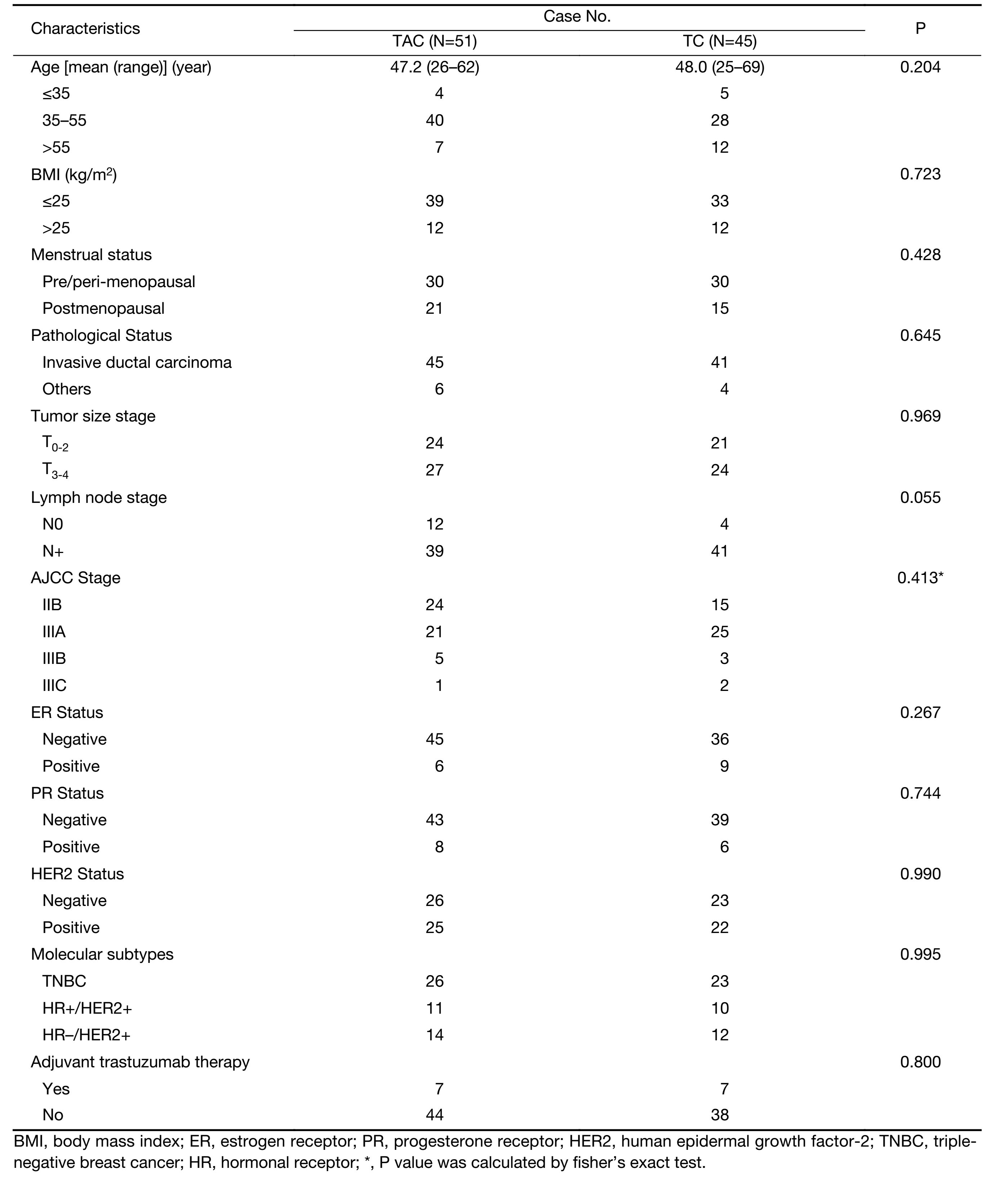
The principal clinical and pathological characteristics were well-balanced between the two treatment arms, which have been described previously (Table 1). Between May 2009 and December 2011, a cohort of 96 patients was enrolled for the current data analysis, of whom 51 received TAC and 45 received TC treatment. The median age was 48.5 years old in the whole population. Fifty-six (58.3%) patients were diagnosed with stage III disease, and 80 (83.3%) patients had clinically positive lymph nodes. Forty-nine patients showed TNBC and 47 cases HER2-positive breast cancer.
Full table
Forty-nine (96.1%) patients were administrated with epirubicin as anthracycline agent. A significant difference in the treatment cycles and dose intensity was not observed between the two arms. After the neoadjuvant treatment and surgery, pCR rate inclined higher in the TAC group than in the TC arm (17.6% vs. 6.8%, P=0.113). The major severe side effects included hematologic toxicities in either group during the neoadjuvant treatment, and the patients recovered well during the long-term follow-up.
Event-free survival outcome
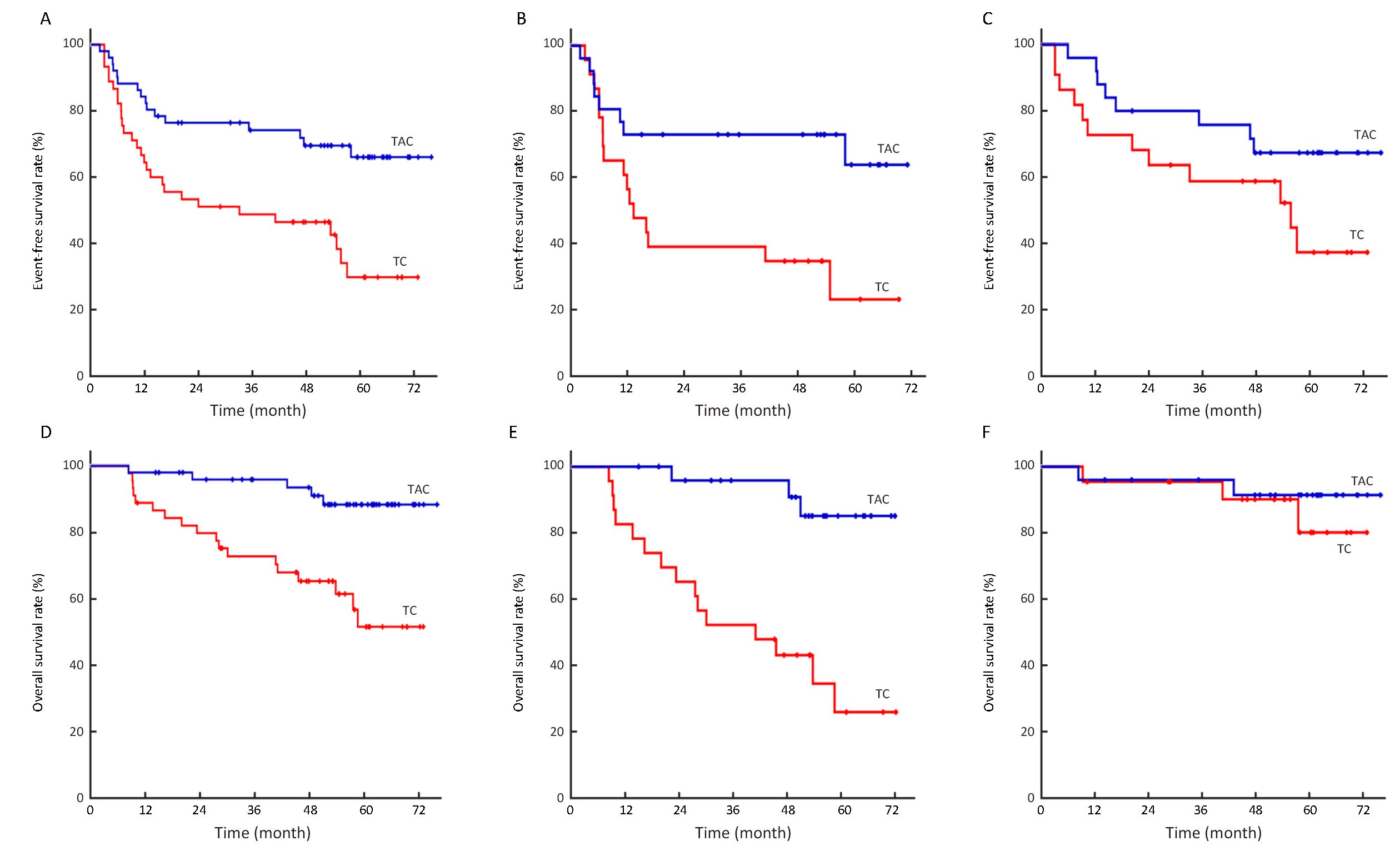
At data cut-off (August 31, 2015), the median follow-up was 52.9 [interquartile range (IQR), 33.7−61.7] months. Fourteen patients received the adjuvant trastuzumab treatment after surgery: 7 cases in each arm. Forty-four patients were recorded to show disease progression, recurrence, or death. Of the 13 patients that had disease progression during the neoadjuvant chemotherapy, 2 patients were diagnosed with contralateral supraclavicular lymph node metastasis and 1 patient was found with bone metastasis. Table 2 shows the disease outcomes according to the neoadjuvant treatment regimens. The events of EFS were found in 28 (62.2%) patients in the TC group, and 16 (31.4%) patients in the TAC group. Eight (15.7%) and 17 (37.8%) patients were diagnosed with distant metastasis in TAC and TC groups, respectively. TNBC or HER2-positive breast cancer patients who received TAC treatment displayed a better EFS than those treated with the TC regimen (HR, 0.39; 95% CI, 0.21−0.73; P=0.002). The estimated 5-year EFS was 66.1% and 29.8% for patients in the TAC and TC groups, respectively, which shows the sustained EFS advantage of adding anthracycline to TC regimen after a longer period of follow-up (Figure 1A). Regarding different molecular subtypes, TAC treatment achieved a better prognosis compared with the TC treatment in TNBC patients (P=0.014, Figure 1B). While in the HER2-positive subtype, 14 patients received adjuvant trastuzumab after surgery, and EFS trend was higher in the TAC arm than the TC arm (P=0.091, Figure 1C).

Full table
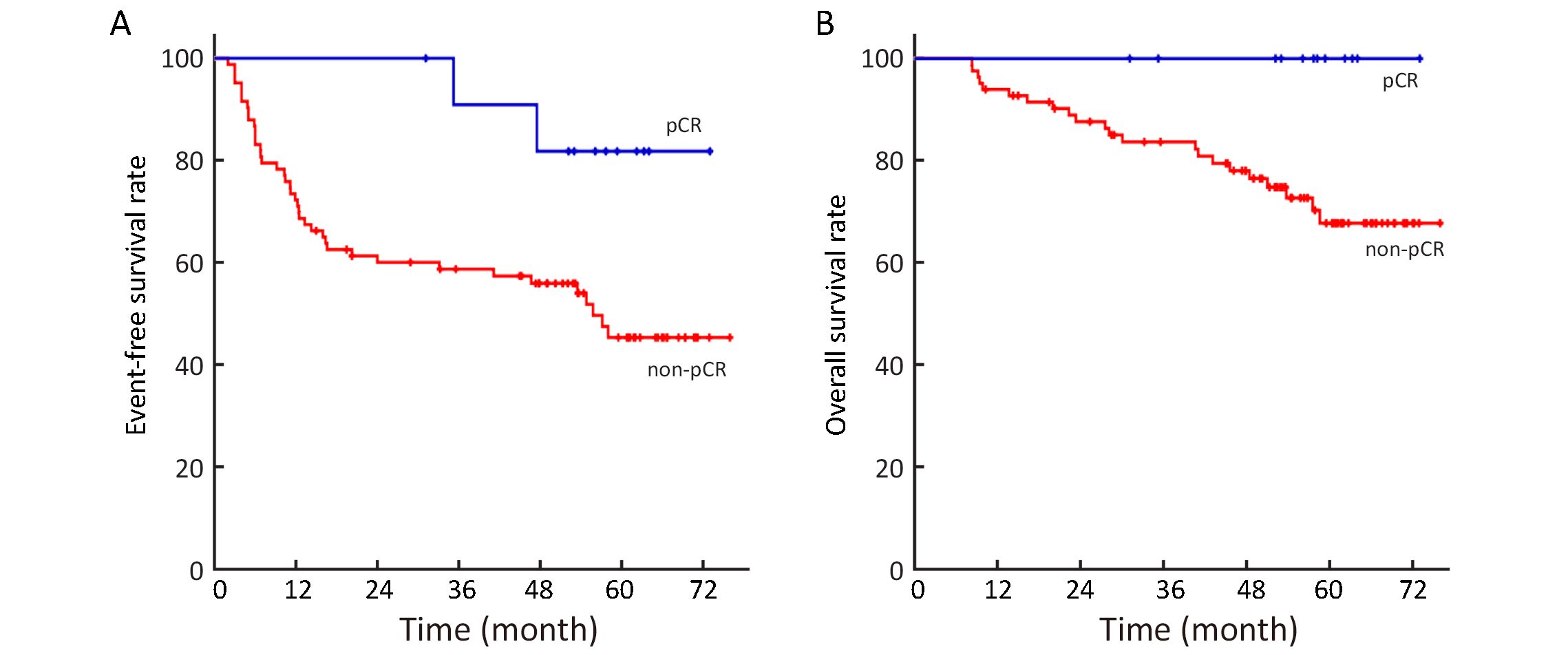
The univariable survival analysis, including age, menopausal status, BMI, clinical tumor size stage, clinical lymph node stage, ER status, PR status, molecular subtype status, pCR status and trastuzumab usage, demonstrated that pCR status and clinical LN stage were associated with EFS outcomes (P=0.035 and P=0.034, respectively). Patients that achieved pCR after neoadjuvant chemo-therapy exhibited satisfactory EFS than those without pCR: the estimated 5-year EFS was 81.8% vs. 45.4% (P=0.035, Figure 2A). The multivariable analysis demonstrated that the neoadjuvant treatment regimen was an independent prognostic factor. After adjusting the factors of pCR status and clinical LN stage, TNBC or HER2-positive breast cancer patients treated with TAC had a superior EFS (HR, 0.48; 95% CI, 0.26−0.90; P=0.021).
Overall survival outcome
During the data cut-off with longer follow-up period, 23 patients perished and 21 were alive after disease pro-gression or recurrence. Fewer deaths occurred in the TAC arm (5 cases) than in the TC (18 cases) (Table 2). The majority of deaths (95.7%) were due to disease recurrence/progression. Kaplan-Meier survival analysis showed that OS was in favor of the TAC treatment; the estimated 5-year OS was 88.4% in the TAC group and 51.6% in the TC group (P<0.001, Figure 1D). In TNBC patients, 18 deaths were recorded, and TAC neoadjuvant treatment was associated with a higher OS as compared to the TC treatment (P<0.001, Figure 1E). Five HER2-positive patients died, and cancer-specific survival at 5 years did not differ significantly between the two treatment arms (P=0.478, Figure 1F).
Any death occurrence was not observed in patients that achieved pCR after the neoadjuvant therapy. The estimated 5-year OS was 67.8% in patients without pCR, which was inferior to those achieved pCR after the neoadjuvant therapy (P=0.049, Figure 2B). In addition to the pCR status and treatment regimen, the univariable analysis also showed that the molecular subtype status was associated with OS (P=0.002). The multivariable analysis, including pCR status, molecular subtype status and treatment regimen, showed that the neoadjuvant treatment regimen and molecular subtype were independently associated with OS. The adjusted HR for OS was 0.20 (95% CI, 0.08−0.60; P=0.003), which was in favor of the TAC neoadjuvant treatment as compared with the TC treatment.
Discussion
After a median follow-up of 53 months, the NATT trial demonstrated a significant EFS and OS benefit from anthracycline-containing TAC neoadjuvant treatment in TNBC and HER2-positive breast cancer patients. In addition, the subgroup analysis showed that the TNBC patients could attain additional benefits from anthracycline-containing neoadjuvant treatment.
Our previous findings showed that TAC was superior to TC regimen regarding EFS with a median follow-up period of 20 months, which led to the premature stop on the study recruitment (11). Consecutively, TAC treatment was numerically better than TC regimen in terms of OS. In the present study, the updated follow-up data demonstrated a sustained EFS benefit from the TAC treatment. Moreover, a total of 23 deaths were recorded, and the TAC neoadjuvant treatment was associated with a significantly improved OS than that for the TC therapy. The joint analysis of ABC trials demonstrated that taxanes plus AC regimens, including TAC, AC followed-up with weekly paclitaxel, or dose-dense paclitaxel, were associated with a better invasive disease-free survival (DFS) than that for the non-anthracycline-containing TC regimen. This result supported anthracycline utility in HER2-negative breast cancer treatment (12).
EBCTCG meta-analysis in 2012 found that 4 standard cycles of AC were equivalent to cyclophosphamide, methotrexate and 5-fluorouracil (CMF) therapy. On the other hand, the higher cumulative dosage of anthracycline could reduce breast cancer mortality as compared to CMF (5). A meta-analysis of Cancer and Leukemia Group B (CALGB) trials showed that ER-negative breast cancer derived more benefits from current adjuvant chemotherapy regimens than ER-positive tumors (13). Also, exploratory analysis from ABC trials found that the additional anthracycline treatment could reduce further disease recurrence in TNBC patients than in the luminal patients (12). In the current study, 18 deaths were recorded in the TNBC patients, which was higher than the HER2-positive patients. This feature may be attributed to the lack of target agents, aggressive biology behavior, and inadequate treatment intensity in TNBC patients (11,14). Moreover, one subgroup of TNBC was immune-related disease and had higher tumor infiltrating lymphocytes, which were associated with a better disease outcome (15). A preclinical study demonstrated that the treatment with anthracycline agents could significantly induce host immunity reaction, which could inhibit the tumor proliferation (16). Our study found that the TNBC patients treated with TAC had superior EFS and OS compared with the TC therapy, indicating that anthracycline is essential for TNBC patients in a neoadjuvant setting.
Several studies have reported that HER2-positive breast cancer has been particularly sensitive to anthracycline treatment (7,8). The efficacy of trastuzumab as neoadjuvant or adjuvant therapy has been well-established in HER2-positive tumors (4). In a neoadjuvant setting, integrating chemotherapy and trastuzumab can significantly increase the pCR rate compared with chemotherapy alone (17). Dual HER2 blockade with additional lapatinib or pertuzumab treatment can further improve the pCR rate (18,19). Currently, chemotherapy plus trastuzumab and pertuzumab regimen has been approved by Food and Drug Administration (FDA) in a neoadjuvant treatment of HER2-positive breast cancer (20). With a median of a 10-year follow-up, Breast Cancer International Research Group (BCIRG) 006 study demonstrated that DFS was similar between TCH and ACTH treatment, supporting that anthracycline may be excluded on the basis of trastuzumab therapy for HER2-positive tumors (21). However, in this NATT study, wherein trastuzumab was not allowed in the neoadjuvant treatment, anthracycline-containing TAC regimen was associated with a trend of higher EFS than the TC treatment in HER2-positive breast cancer patients. OS was not significantly different between these two treatment arms. This phenomenon may potentially be explained by 14 patients receiving adjuvant trastuzumab treatment, a relatively small number of enrolled patients, and available effective anti-HER2 target agents for patients with metastatic disease (22).
In the neoadjuvant treatment, both Germany and FDA meta-analysis demonstrated that pCR could predict the survival outcome in TNBC or HER2-positive breast cancer patients (4,23). In this study, TNBC or HER2-positive breast cancer patients who achieved pCR had a significantly improved EFS and OS than those without pCR. NeOAdjuvant Herceptin (NOAH) study demonstrated that the doubled pCR rate with neoadjuvant trastuzumab treatment can translate into EFS benefit in HER2-positive tumors (17). For TNBC patients, adding carboplatin to anthracycline-and taxane-containing regimen significantly improved the pCR rate (24), which was also associated with numerically higher EFS (25). Findings from our previous NATT study showed that the pCR rate difference was not apparent between the TAC and TC treatment, whereas additional disease progressions were observed during neoadjuvant therapy in the TC group. Retrospective data showed that TNBC with residual disease after neoadjuvant chemotherapy exhibited worse DFS compared with the other breast cancer subtypes (26). In the current study, the updated follow-up data demonstrated not only a sustained EFS benefit but also a better OS outcome from additional anthracycline treatment, especially in TNBC patients. This feature may arise from a relatively low pCR rate and more patients with residual diseases after neoadjuvant chemotherapy in this cohort of patients.
Conclusions
With a median follow-up period of 53 months, anthra- cycline-containing TAC neoadjuvant treatment had a sustained EFS benefit and a better OS compared with the TC treatment in TNBC and HER2-positive breast cancer patients. This updated follow-up results postulate that anthracycline should be considered as a necessary and effective drug in this trial setting, especially in TNBC patients.
Acknowledgements
The authors thank all patients and family members for their willingness to enter into this study. The authors also acknowledge the following NATT investigators who enrolled and followed patients for this study from China: Yiding Chen (Zhejiang), Xiaohong Xie (Zhejiang), Hong Zheng (Sichuan), Yali Cao (Jiangxi), Kejin Wu (Shanghai), Duo Ni (Xinjiang), Jinhai Tang (Jiangsu), Ziguo Wei (Shandong), Yuechu Dai (Zhejiang), Xiaohua Zhang (Zhejiang), Huifang Hu (Jiangsu), Dedian Chen (Yunnan), Hongwei Zhang (Shanghai), Xiaodong Wang (Jiangsu), Jianjun He (Shanxi), Quchang Ouyang (Hunan), Weili Wu (Zhejiang), Anqin Zhang (Guangdong), Qing Shao (Jiangsu), Hui Song (Shanghai), Qi He (Shanghai), Yu Guo (Zhejiang), and Lili Tang (Hunan).
Funding: This investigator sponsored trial was supported in part by the grants from the National Natural Science Foundation of China (Grant No. 81472462), Medical Guidance Foundation of Shanghai Municipal Science and Technology Commission (Grant No. 15411966400), Technology Innovation Act Plan of Shanghai Municipal Science and Technology Commission (Grant No. 14411950200, 14411950201) and Sanofi.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chia S, Swain SM, Byrd DR, et al. Locally advanced and inflammatory breast cancer. J Clin Oncol 2008;26:786–90. [PubMed] DOI:10.1200/JCO.2008.15.0243
- Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883–91. [PubMed] DOI:10.1200/JCO.2014.55.2836
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemo-therapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. [PubMed] DOI:10.1200/JCO.2011.38.8595
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies — improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46. [PubMed] DOI:10.1093/annonc/mdv221
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. [PubMed] DOI:10.1016/S0140-6736(11)61625-5
- Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177–83. [PubMed] DOI:10.1200/JCO.2008.18.4028
- Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 2008;100:14–20. [PubMed] DOI:10.1093/jnci/djm252
- Cheang MC, Voduc KD, Tu D, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res 2012;18:2402–12. [PubMed] DOI:10.1158/1078-0432.CCR-11-2956
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83. [PubMed] DOI:10.1056/NEJMoa0910383
- Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol 2016;13:172–84. [PubMed] DOI:10.1038/nrclinonc.2015.171
- Chen X, Ye G, Zhang C, et al. Superior outcome after neoadjuvant chemotherapy with docetaxel, anthracycline, and cyclophosphamide versus docetaxel plus cyclophosphamide: results from the NATT trial in triple negative or HER2 positive breast cancer. Breast Cancer Res Treat 2013;142:549–58. [PubMed] DOI:10.1007/s10549-013-2761-1
- Blum J, Flynn P, Yothers G, et al. Interim joint analysis of the ABC (anthracyclines in early breast cancer) phase III trials (USOR 06-090, NSABP B-46I/USOR 07132, NSABP B-49 [NRG Oncology]) comparing docetaxel + cyclophosphamide (TC) v anthracycline/taxane-based chemotherapy regimens (TaxAC) in women with high-risk, HER2-negative breast cancer. J Clin Oncol 2016;34(suppl):abstr 1000.
- Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 2006;295:1658–67. [PubMed] DOI:10.1001/jama.295.14.1658
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674–90. [PubMed] DOI:10.1038/nrclinonc.2016.66
- Mao Y, Qu Q, Chen X, et al. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One 2016;11:e0152500. [PubMed] DOI:10.1371/journal.pone.0152500
- Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016;13:228–41. [PubMed] DOI:10.1038/nrclinonc.2015.215
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377–84. [PubMed] DOI:10.1016/S0140-6736(09)61964-4
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633–40. [PubMed] DOI:10.1016/S0140-6736(11)61847-3
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. [PubMed] DOI:10.1016/S1470-2045(11)70336-9
- Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res 2014;20:5359–64. [PubMed] DOI:10.1158/1078-0432.CCR-14-1268
- Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of the BCIRG-006 Trial comparing doxorubicin plus cyclophosphamide followed by docetaxel (ACT) with doxorubicin plus cyclo- phosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer patients. San Antonio Breast Cancer Symposium 2015:S5–04.
- Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461–71. [PubMed] DOI:10.1016/S1470-2045(13)70130-X
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. [PubMed] DOI:10.1016/S0140-6736(13)62422-8
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747–56. [PubMed] DOI:10.1016/S1470-2045(14)70160-3
- von Minckwitz G, Loibl S, Schneeweiss A, et al. Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neo-adjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). San Antonio Breast Cancer Symposium 2015:S2–04.
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–81. [PubMed] DOI:10.1200/JCO.2007.14.4147
