CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial
Introduction
Colon cancer (CC) is one of the most common malignancies worldwide and the leading cause of cancer death in women and men worldwide (1). In western countries, CC is the second leading cause of cancer death (2). Recent reports from the World Health Organization (WHO) show that the incidence of colon cancer is rising rapidly in many Asian countries (3). In China, combined with rectal cancer (RC), CC is the fifth leading cause of cancer-associated mortality and the proportion is even higher in much more developed cities such as Shanghai (4).
Despite recent development in treatment, locally advanced CC (LACC) represents a major therapeutic challenge (5,6), which is defined as tumors in T3 stage with ≥5 mm invasion beyond the muscularis propria or T4 (penetration within adjacent organs) staging by computed tomography (CT) scan (7,8). Further in ECKINOXE trial conducted by Karoui et al., LACC was defined as high-risk T3 (disruption of muscle wall and extension into pericolic fat with more than 5 mm protrusion into adjacent mesenteric fat)-T4 (penetration within adjacent organs) and/or N2 (more than 3 clustered lymph nodes above 1 cm in shortest diameter) (9). Radical surgery followed by adjuvant chemotherapy is recommended for the patients with LACCs (10). However, 20%−30% of patients with stage II/III CCs developed local or distant recurrences, which indicates the ineffectiveness of the treatment in eradicating regional spread and distant micrometastases.
Recently several studies showed that neoadjuvant chemotherapy (NAC) is an effective treatment option for aggressive solid tumors including resectable and unresectable tumors, such as gastroesophageal cancer, gastric cancer and breast cancer (11-14). It is thought to confer an advantage over other treatment regiments through the eradication of potential micrometastases or circulating tumor cells; reduction of surgical tumor cell shedding; improvement of complete resection rate owing to primary tumor regression; maintenance of chemotherapy intensity; evaluation of chemotherapy safety and chemosensitivity, thereby contributing to the assessment of the need for postoperative chemotherapy or selection of the appropriate chemotherapy regimen; and the circumvention of delayed postoperative chemotherapy owing to surgical complications (14-17).
On the other hand, the potential disadvantages associated with NAC include the risk of overtreatment due to inaccurate radiological staging, leading to severe toxicity in low-risk patients; risk of bowel obstruction or perforation caused by the primary tumor during preoperative treatment, resulting in emergency but not radical surgery; increased risk of perioperative complications; prolonged hospital stay and increased fees; delayed adjuvant chemotherapy; and tumor progression during NAC.
To date, NAC has not been routinely administered in patients with operable LACCs. There were only two prospective studies about oxaliplatin plus capecitabine (CapOX) without target treatment as NAC regimen for LACCs (NCT01675999-Paris and NCT02572141-Guangzhou) in progress and no results were available. The latest published study about NAC applied to the LACCs was a retrospective study and the result showed that NAC for operable LACC patients was safe and able to induce major tumor regression (18).
Based on the situations above, the efficacy and safety of CapOX as NAC regimen for LACCs were needed to identify. The primary objective of this phase II study was to evaluate the efficacy and safety of CapOX as a neoadjuvant treatment for patients with operable LACCs.
Materials and methods
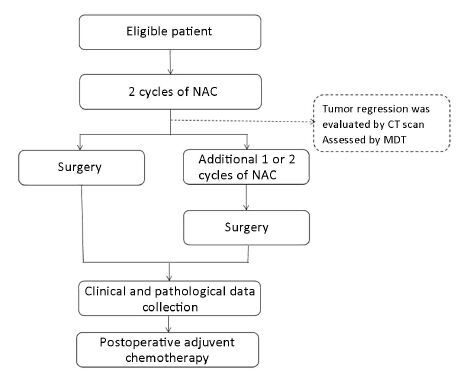
The study was a prospective single-arm phase II study conducted in a single center in China. We evaluated the safety and efficacy of preoperative CapOX regimen in patients with locally advanced CCs. This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center and written informed consent was obtained from all participants. The trial is registered in ClinicalTrials.gov (No. NCT02415829). The trial protocol is shown in Figure 1.
Patients
Inclusion criteria
The inclusion criteria were: 1) pathologically confirmed colon carcinoma; 2) CT-defined T4 or lymph node-positive resectable CC; 3) no history of previous treatment; 4) obstructive CC treated with defunctional stoma or exploratory laparotomy; 5) age ≥18 years and ≤75 years; 6) Eastern Cooperative Oncology Group (ECOG) performance status 0−1; 7) no prior chemotherapy or abdominal or pelvic irradiation; 8) life expectancy ≥3 months; 9) no history of CRC; and 10) laboratory analysis showing leukocytes ≥3×109/L with neutrophils ≥1.5×109/L, platelets ≥100×109/L, hemoglobin ≥9 g/dL (5−6 mmol/L), total bilirubin ≤1.5×upper limit of normal (ULN), aspartate aminotransterase (ASAT) and alanine aminotransferase (ALAT) ≤2.5×ULN, alkaline phosphatase ≤1.5×ULN, and serum creatinine ≤1.5×ULN.
Exclusion criteria
The exclusion criteria were: 1) tumors within 15 cm of the anal verge determined by sigmoidoscopy, or below the sacral promontory determined by imaging; 2) evidence of distant metastases or peritoneal carcinomatosis by CT scan; 3) colonic obstruction without prior defunctional stroma or stent treatment, or 4) serious medical comorbidity that might hinder neoadjuvant therapy and/or surgery.
Treatment
The NAC consisted of 3 cycles of CapOX (130 mg/m2 of intravenous oxaliplatin on d 1, plus 1,000 mg/m2 of oral capecitabine twice daily on d 1−14, repeated at 3 week intervals). Dose reduction and up to 4 weeks of delay were allowed in case of reversible toxicity.
CT scan assessed tumor regression after 2 cycles of NAC. If progressive or stable disease was detected, surgery was scheduled immediately. Otherwise, surgery was scheduled after additional 1−2 cycles of chemotherapy. All the patients' conditions were assessed by multiple disciplinary teams (MDT). In order to reduce perioperative morbidity, surgery must be performed at least 1 week after the completion of preoperative chemotherapy. Both laparoscopic and open surgeries were considered. Following surgery, the rest cycles of chemotherapy were administered using a schedule identical to that of NAC, and each patient received 8 cycles of chemotherapy in all.
Efficacy and safety assessment
Clinical response assessment
The initial evaluation included medical history, clinical examination, complete laboratory analysis, metastatic evaluation [(by chest CT, abdominal and pelvic enhanced CT or magnetic resonance imaging (MRI)], colonoscopy and tumor biopsy. The response to treatment was assessed by physical examination after the completion of 2 cycles of CapOX treatment. The clinical responses following CapOX treatment were evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 guideline (RECIST version 1.1).
Histological response assessment
Pathologic response to chemotherapy was determined by examination of the primary tumor and lymph node specimens collected during surgery by two independent pathologists. The pathological response of each primary tumor was scored according to the American Joint Committee on Cancer staging criteria. This scoring determined the tumor regression grade (TRG) depending on the presence of residual tumor cells and the extent of fibrosis. The definitions for TRG 0−3 were no residual tumor cells, single cells or small group of cells, residual cancer with desmoplastic response and minimal evidence of tumor response, respectively (19).
Safety assessment
Toxicity and adverse events (AEs) were described according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.02 (CTCAE version 4.02).
Endpoints
The primary endpoint of the study was pathological response indicated by TRG. The secondary endpoints were the frequency, severity, and attribution of AEs associated with neoadjuvant treatment, technical difficulty of surgery using both subjective (e.g., perceived technical difficulty of the operation by the surgeon on an arbitrary scale) and objective (e.g., estimated blood loss, time of operation, and intraoperative complications) criteria, and the frequency and severity of surgical complications. The primary and secondary endpoints were measured within the 30-d postoperative period.
The patients were followed up every 3 months in the first two years. From the third year, follow-up was scheduled every 6 months.
Results
Patient characteristics
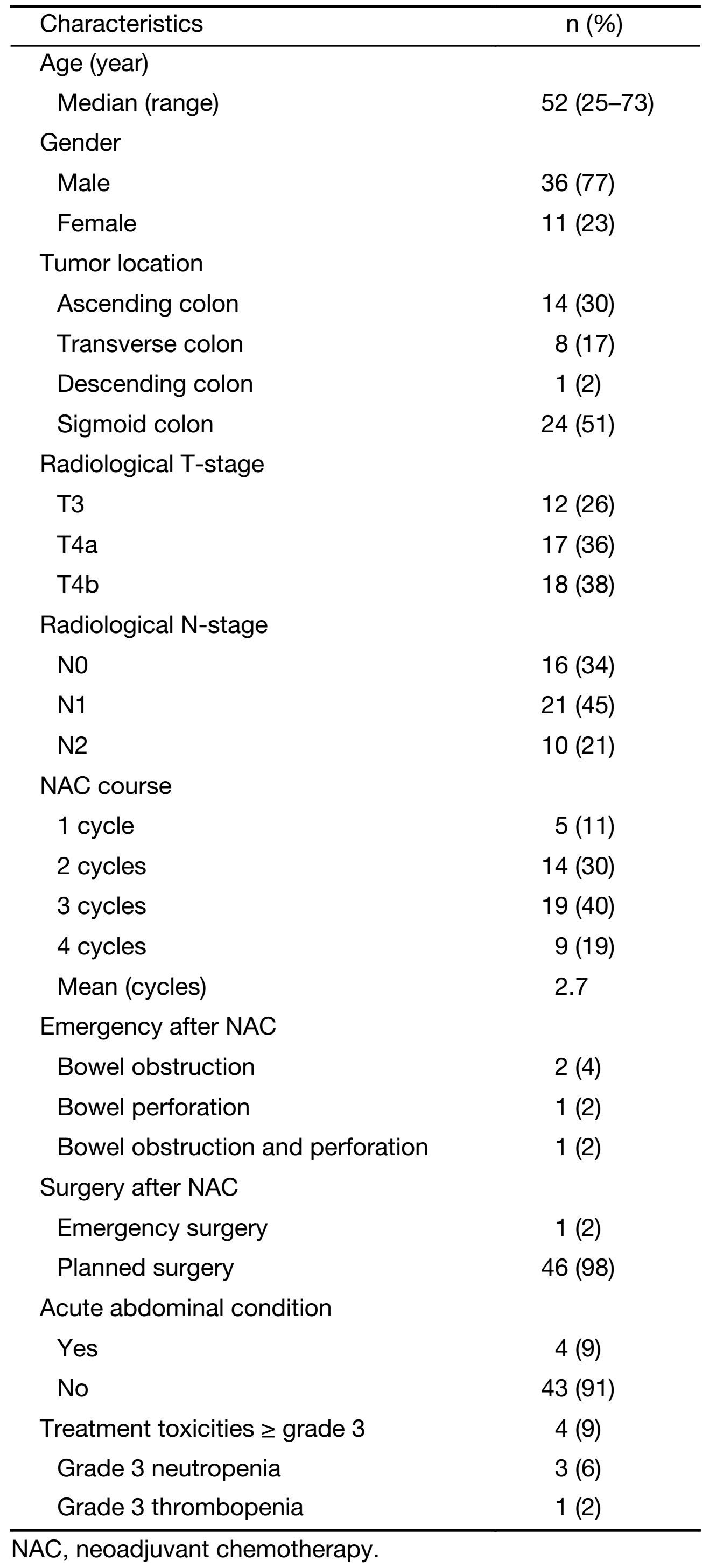
In total, 47 patients were enrolled between February 2015 and June 2016. All patients were eligible for study inclusion and were treated with curative intent. Patients' characteristics are summarized in Table 1.
Full table
Chemotherapy
Forty-two patients completed the planned treatments. The mean NAC course was 2.7 cycles. Five patients only received 1 cycle of NAC. Four of them opted for surgery immediately and another one developed perforation after NAC.
NAC-related AEs
The AEs associated with NAC were few and well tolerated. Only 4 patients developed grade 3 hematological adverse reactions after chemotherapy including grade 3 neutropenia (n=3) and grade 3 thrombopenia (n=1). There was no chemotherapy-induced grade 4 AE. In addition, most AEs, such as gastrointestinal discomfort and myelosuppression, were mild and treated for symptoms. There was no treatment-related death.
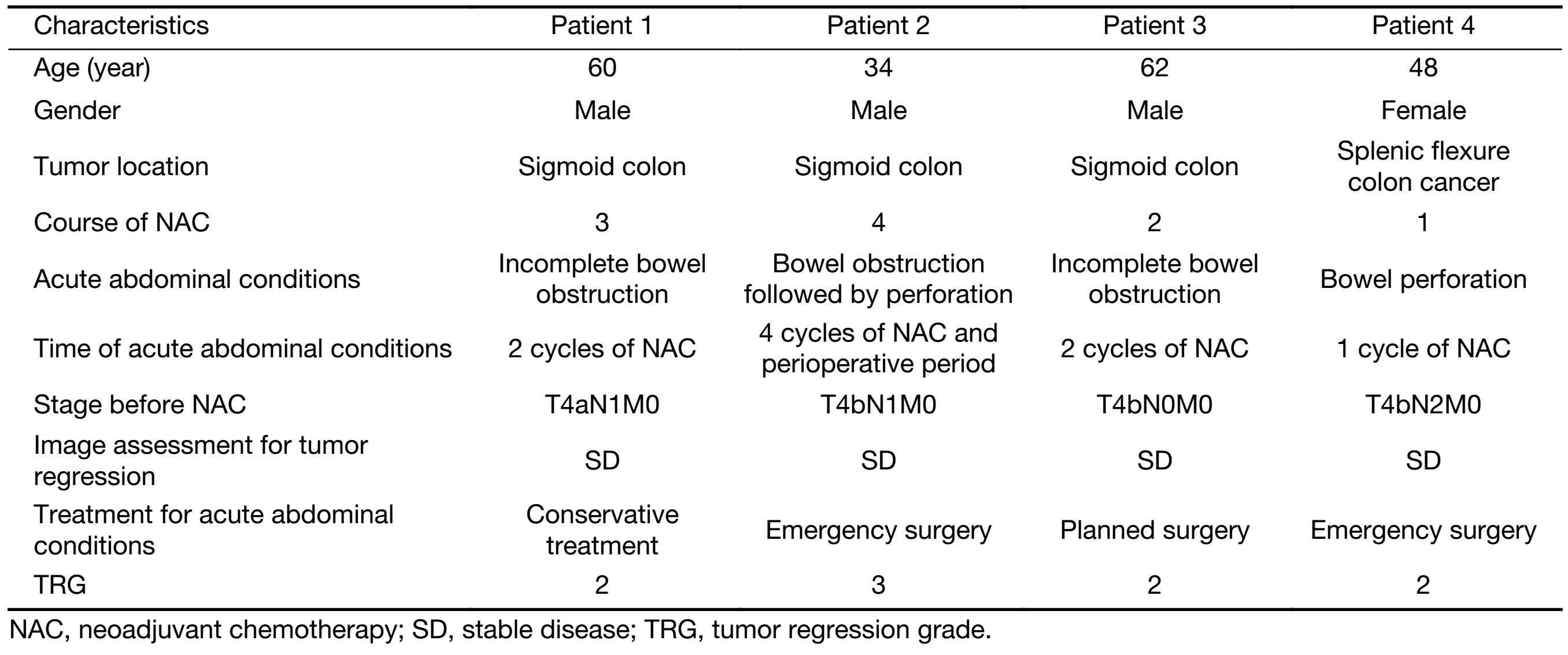
Acute abdominal conditions developed in 4 (4/47, 9%) patients after NAC including bowel obstruction and perforation. Only one patient did not complete the NAC and received emergency surgery. The first patient showed symptoms of bowel obstruction after 2 cycles of NAC and another 1 cycle of NAC was given after treated with conservative treatment. The TRG was 2. The second patient was admitted to hospital after 4 cycles of NAC and surgery was prepared according to the treatment plan. But Bowel obstruction followed by perforation occurred in the planned preoperative preparation stage. The TRG was 3. The third patient developed incomplete obstruction after 2 cycles of NAC and was admitted to hospital for surgery. The TRG was 2. Bowel perforation occurred in the fourth patient after 1 cycle of NAC and surgery was immediately scheduled for her. The TRG was 2. No postoperative complications occurred in all the patients above (Table 2).
Full table
Surgery
All patients underwent surgery including planned surgery and acute surgery (46 vs. 1, respectively). All patients who underwent surgery achieved pathological complete resection (R0 resection). Postoperative complications were observed in 1 patient with wound infection (1/47, 2%). The mean postoperative stay in the hospital was 9.4 d and there was no 30-d operative mortality. All of the patients received planned post- operative adjuvant chemotherapy.
Clinical response and pathological results
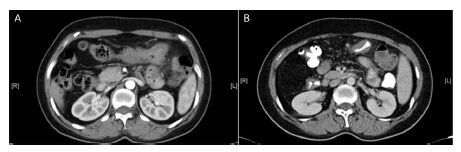
All the patients received both clinical and pathological assessment of tumor regression after NAC. Planned treatment was completed by 89% of patients (42/47). The total response rate was 68% (32/47), including complete and partial response rates of 2% (1/47) and 66% (31/47), respectively. Stable disease was observed in 32% (15/47) of the patients and progressive disease was observed in none. The tumor regression assessed by CT scan for one of the patients is shown in Figure 2.
The TRG score assessed the total pathological response rate. Complete pathologic response (TRG 0), major regression (TRG 1), and at least moderate regression (TRG 2) were achieved in 1 (2%), 2 (4%), and 29 (62%) patients, respectively (Table 3).
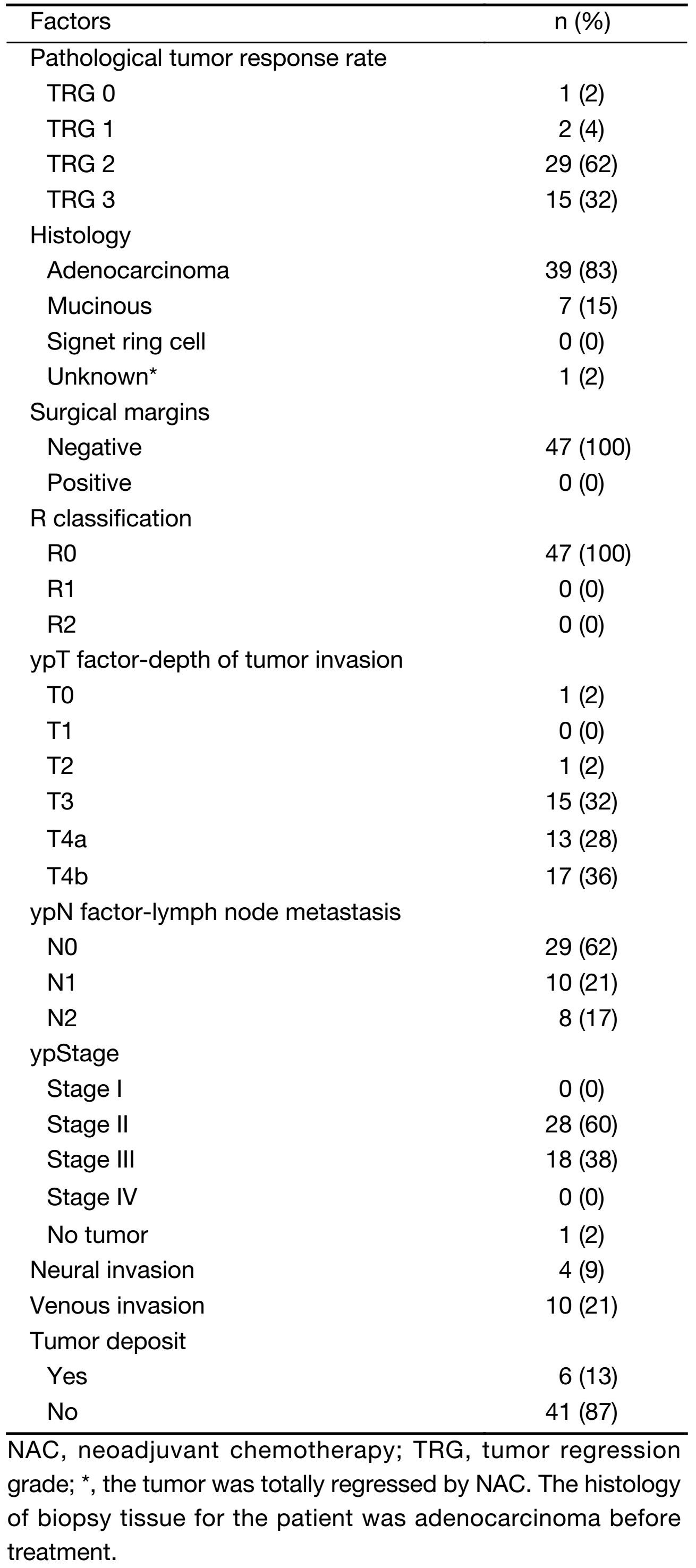
Full table
Discussion
Presently, the standard therapeutic approach for non-metastatic CC is surgery and/or adjuvant chemotherapy. By contrast, more treatment strategies are available for rectal cancer, particularly for locally advanced rectal cancer. For example, NAC for rectal cancer improved local control and might influence long-term survival of patients with rectal cancer (20).
Neoadjuvant treatment has been effective in the treatment of several malignant tumors and is part of the standard treatment for breast, esophageal, and gastric cancers (11-14,21). In locally advanced disease, preoperative chemotherapy might reduce tumor size, eradicate micrometastases, and improve operability and surgical downstaging (14-17).
NAC has recently received considerable attention as a treatment strategy most likely improving survival outcomes of patients with advanced CRCs. The FOxTROT trial was the first to evaluate the effectiveness of preoperative chemotherapy in locally advanced CC (8). Patients were randomly assigned to preoperative chemotherapy followed by surgery and a further nine cycles of OxMdG or standard surgery followed by postoperative chemotherapy. Patients with KRAS wild-type tumors were randomly assigned to receive panitumumab or not. The results of the FOxTROT study showed that preoperative chemotherapy for locally advanced operable primary CC is feasible, with tolerable toxicity and perioperative morbidity. On the basis of these promising findings from the pilot phase, the FOxTROT phase III study is ongoing to investigate the long-term oncological outcomes.
Another study with available results was conducted by Jakobsen et al. and published in 2015 (15). The NAC regimen of the study was neoadjuvant chemotherapy followed by surgery and the postoperative chemotherapy was chosen according to the pathological result. Patients with KRAS wild-type tumors received chemotherapy combined with panitumumab. Conversion rate expressed as the fraction of patients not fulfilling the criteria for adjuvant chemotherapy was the primary endpoint. The results showed that the conversion rates in wild-type and mutated (including unknown) patients were 42% and 51%, respectively. The study showed that NAC in colon cancer was feasible and the results indicated that a major part of the patients can be spared adjuvant chemotherapy. A multicenter randomized phase III trial was conducted and the NAC regimen was CapOX followed by surgery or standard surgery (NCT01918527). To date, there were no available results about the trial.
In addition, there were another two clinical trials about NAC of CC (NCT01675999-Paris and NCT02572141-Guangzhou). The two studies were still in progress without available results.
The latest research on NAC of CC was published in August 2016 (18). It was a retrospective study conducted by Arredondo et al. Sixty-five LACC patients were included and the results showed that NAC in LACC patients was safe and able to induce major tumor regression. However, it was a retrospective study and further research was warranted.
Although there are several studies about NAC for CC, several issues around the use of NAC for CC still remain. One of the concerns is the choice of the NAC regimen. We can know from the above studies that there was no standard regimen for NAC and the optimal preoperative chemotherapy strategy was still unknown. OxMdG+/-panitumumab, CapOX+/-panitumumab and FOLFOX+/-C225 were chosen in clinical trials respectively (8,9,15,18).
CapOX and FOLFOX are the standard treatment for high-risk stage II and stage III CC, and the first-line chemotherapy in patients with stage IV CCs (10,16,22-24). Due to lower frequency than FOLOFX and easier use except for efficacy, the CapOX regimen was widely used for adjuvant chemotherapy in non-metastasis CRC patients. For targeted therapy, there was no enough evidence to confirm the efficacy for non-metastasis CCs. The current results showed that there were no signs of increased conversion rate in the patients receiving NAC and panitumumab than patients receiving NAC only (15). In addition, in China, not every patient with LACCs could receive the targeted therapy due to the cost of expensive medical bills paid by patients themselves.
Based on the situations above, the CapOX regimen potentially became the ideal treatment choice of NAC for LACCs in China. The primary objective of this phase II study was to evaluate the efficacy and safety of CapOX as a neoadjuvant treatment for patients with locally advanced CCs.
Our study is a prospective phase II trial with available results investigating the efficacy and safety of CapOX regimen as NAC without targeted therapy for patients with LACCs, and demonstrated that CapOX as NAC regimen was safe, effective and well completed for patients with LACCs. The completion rate was 89%. The most frequently observed toxicities in the present study were hematological toxicities and gastrointestinal discomfort. Only 4 patients developed grade 3 hematological adverse reactions after chemotherapy. There were no severe AEs during NAC. All of the toxicities that developed were well tolerable and manageable.
In this study, all the patients with bowel obstruction were not caused by tumor progression. All the tumors presented some degree of regression which was assessed by the postoperative pathological tumor regression score. There were some interpretations for bowel obstruction as follows: bowel contraction was caused by tumor regression after NAC and a large number of stool accumulations during the preoperative bowel preparation. During the operation, the tumor specimen with scar can be found and it indirectly identified that the tumor regression with scar was the reason for bowel obstruction.
In our study, both clinical and pathological responses were assessed. The clinical response rate was 68%, which was consistent with pathological response rate. The results identified the efficacy of NAC with CapOX. Further, these observations confirmed the importance of clinical assessment and suggested that careful abdominal enhanced CT could be a relatively reliable pathological response predictor for NAC.
The accuracy of clinical staging via imaging before surgery is crucial to ensuring that patients without the requirement of NAC do not get over-treated. Recent advances in radiology permit better prediction of tumor stage (wall penetration and nodal involvement) before surgery (25). The features of stage T4 cancer on CT include nodular penetration of the tumor through the peritonealized areas of the muscle coat, or an advancing edge of the tumor penetrating adjacent organs. Peritoneal infiltration identified by CT can be used in the classification of patients as stage T4 and high risk.
In addition, some studies indicated that NAC could be associated with the survival outcome of patients, and the achievement of pathological complete remission (pCR) using NAC correlated with disease-free survival. This is considered as an early indicator of a novel regimen with good efficacy (26). Although the main purpose of the present study was to assess the efficacy and safety of CapOX as NAC in the treatment of CC and the prognosis was not the aim of the study, we still followed up the patients' prognosis. Our results could not identify whether the curative effect of NAC is better than that of traditional surgery because it was a single arm phase II study. However, the outcome of patients was inspiring, and data on long-term survival will be collected in the future research.
Conclusions
In conclusion, the present study demonstrated that the CapOX regimen showed high R0 resection rate and high response rate (clinical and pathological) in the treatment of locally advanced CC. Thus, CapOX was proved to be a safety and efficacious treatment strategy with moderate toxicity. Nevertheless, the long-term follow-up is required to translate these findings to improve local control and overall survival. We plan to confirm the result of this study in a future phase III trial.
Acknowledgements
We thank the patients and all investigators.
Funding: This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81472620); Shanghai Science and Technology Planning Fund (No. 13140902100); Shanghai Combination Study Project for Major Diseases (No. 2014ZYJB0101); and Shanghai Health and Family Planning Commission (No. JGY1404).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [PubMed] DOI:10.3322/caac.21254
- Belot A, Grosclaude P, Bossard N, et al. Cancer incidence and mortality in France over the period 1980-2005. Rev Epidemiol Sante Publique 2008;56:159–75. [PubMed] DOI:10.1016/j.respe.2008.03.117
- Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull 2013;105:29–42. [PubMed] DOI:10.1093/bmb/lds040
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.01.06
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation — technical notes and outcome. Colorectal Dis 2009;11:354–64. [PubMed] DOI:10.1111/cdi.2009.11.issue-4
- Kontovounisios C, Tan E, Pawa N, et al. Selection process can improve the outcome in locally advanced and recurrent colorectal cancer: activity and results of a dedicated multidisciplinary colorectal cancer centre. Colorectal Dis 2016. [Epub ahead of print]
- Smith NJ, Bees N, Barbachano Y, et al. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer 2007;96:1030–6. [PubMed] DOI:10.1038/sj.bjc.6603646
- Foxtrot Collaborative Group. Feasibility of pre-operative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152–60. [PubMed] DOI:10.1016/S1470-2045(12)70348-0
- Karoui M, Rullier A, Luciani A, et al. Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a multicentre randomised controlled phase II trial — the PRODIGE 22 — ECKINOXE trial. BMC Cancer 2015;15:511. [PubMed] DOI:10.1186/s12885-015-1507-3
- Varghese A. Chemotherapy for stage II colon cancer. Clin Colon Rectal Surg 2015;28:256–61. [PubMed] DOI:10.1055/s-00000049
- Lerebours F, Rivera S, Mouret-Reynier MA, et al. Randomized phase 2 neoadjuvant trial evaluating anastrozole and fulvestrant efficacy for post-menopausal, estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients: Results of the UNICANCER CARMINA 02 French trial (UCBG 0609). Cancer 2016;122:3032–40. [PubMed] DOI:10.1002/cncr.v122.19
- Samalin E, Ychou M. Neoadjuvant therapy for gastroesophageal adenocarcinoma. World J Clin Oncol 2016;7:284–92. [PubMed] DOI:10.5306/wjco.v7.i3.284
- Yoshikawa T, Morita S, Tanabe K, et al. Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of S-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer 2016;62:103–11. [PubMed] DOI:10.1016/j.ejca.2016.04.012
- Wang X, Zhao L, Liu H, et al. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer 2016;114:1326–33. [PubMed] DOI:10.1038/bjc.2016.126
- Jakobsen A, Andersen F, Fischer A, et al. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol 2015;54:1747–53. [PubMed] DOI:10.3109/0284186X.2015.1037007
- Suenaga M, Fujimoto Y, Matsusaka S, et al. Perioperative FOLFOX4 plus bevacizumab for initially unresectable advanced colorectal cancer (NAVIGATE-CRC-01). Onco Targets Ther 2015;8:1111–8. [PubMed] DOI:10.2147/OTT.S83952
- Bayraktar UD, Chen E, Bayraktar S, et al. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma?. Cancer 2011;117:2364–70. [PubMed] DOI:10.1002/cncr.25720
- Arredondo J, Baixauli J, Pastor C, et al. Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemo-herapy and surgery. Clin Transl Oncol 2016. [Epub ahead print]
- Trakarnsanga A, Gnen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 2014; 106. pii: dju248.
- Dueland S, Ree AH, Grholt KK, et al. Oxaliplatin-containing preoperative therapy in locally advanced rectal cancer: local response, toxicity and long-term outcome. Clin Oncol (R Coll Radiol) 2016;28:532–9. [PubMed] DOI:10.1016/j.clon.2016.01.014
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [PubMed] DOI:10.1056/NEJMoa055531
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015;33:4176–87. [PubMed] DOI:10.1200/JCO.2015.63.4238
- Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–42. [PubMed] DOI:10.1001/jama.2011.749
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51. [PubMed] DOI:10.1056/NEJMoa032709
- Burton S, Brown G, Bees N, et al. Accuracy of CT prediction of poor prognostic features in colonic cancer. Br J Radiol 2008;81:10–9. [PubMed] DOI:10.1259/bjr/19492531
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemo-therapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. [PubMed] DOI:10.1200/JCO.2011.38.8595