A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer
Introduction
Colon cancer is a main cause of cancer-related death throughout the world, and the standard treatment for locally advanced resectable colon cancer, including stage III and high-risk stage II disease, is surgery followed by postoperative adjuvant chemotherapy (1-3). Although therapeutics have been improved in the last decades, some patients ultimately develop metastatic disease with a 5-year disease-free survival (DFS) of 69%–73% (4,5).
Neoadjuvant chemotherapy has many advantages and is widely utilized in gastric, breast and bladder cancer (6-8). In certain stages of the disease, it is substantially more effective than postoperative therapy (9). In recent years, neoadjuvant chemotherapy has been introduced into treatment for resectable metastatic and locally advanced colon cancer (10-14). In a pilot II trail, the feasibility, safety and efficiency with the same perioperative morbidity, acceptable toxicity and significant tumor down-staging of preoperative chemotherapy as the standard UK modified de Gramont (OxMdG) regimen were demonstrated (14).
The FOLFOXIRI regimen combines all the three active chemotherapeutic agents (irinotecan, oxaliplatin, 5-fluorouracil and leucovorin), and has higher response rate (RR), and better progression-free survival (PFS) and overall survival (OS) than two-agent regimens in metastatic colorectal cancer (15-18). Neoadjuvant treatment is expected to have a significant effect on tumor-regression and down-staging, and can markedly shrink tumor in limited cycles. Thus, the surgery following neoadjuvant treatment could remove the tumor more completely and radically. Theoretically, FOLFOXIRI will be more suitable for the demand of neoadjuvant chemotherapy. With significant RR improvement, the FOLFOXIRI regimen also increases the rate of grade 3−4 toxicity, such as neutropenia and diarrhea (17-19). Will the increased toxicity impact safety following operation and result in higher morbidity rate? In the present prospective phase II study, the safety and efficacy of the FOLFOXIRI regimen in the neoadjuvant treatment of resectable locally advanced colon cancer are discussed.
Patients and methods
Study design
This is a prospective, open-label single arm phase II study. The protocol was approved by the Ethical Committee of Cancer Hospital (Institute), Chinese Academy of Medical Science (Approval number 14−032/822). The study was conducted in accordance with the principles of the Declaration of Helsinki and the note for guidance on good clinical practice. The trial is registered in ClinicalTrials.gov (No. NCT02688023).
Main eligibility criteria
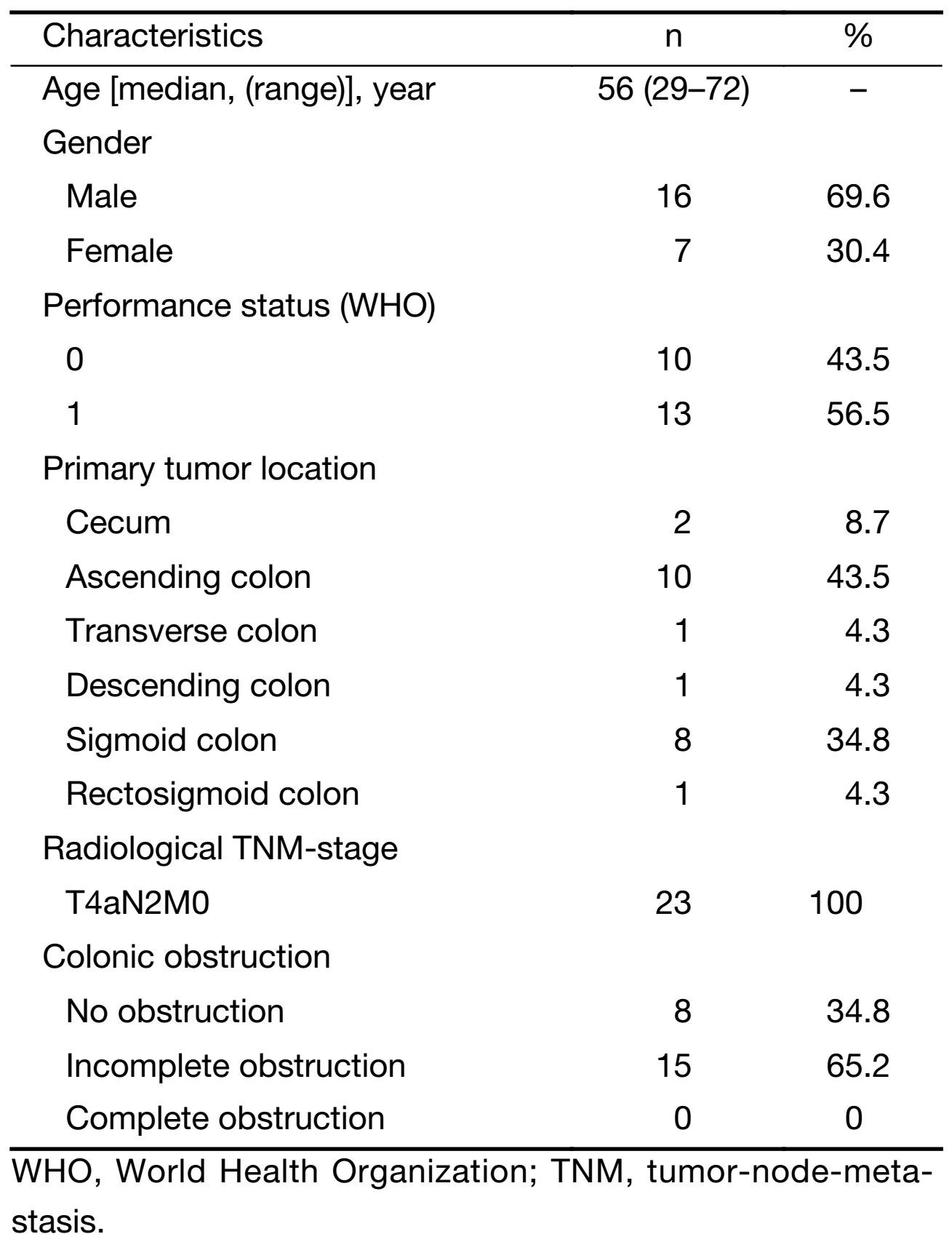
Patients recruited had histologically verified colon cancer at clinical stage T4N2M0 [American Joint Committee on Cancer (AJCC) TNM classification 2009]. Clinical T4 stage was defined as no slit between the serosa and tumor or serosal penetration of tumor while clinical N2 stage meant more than 3 lymph nodes with contrast enhanced and short diameter longer than 1 cm on computed tomography (CT) scan. Other inclusion criteria were: 1) 18−70 years of age at diagnosis; 2) World Health Organization (WHO) performance status ≤2; 3) adequate bone marrow, liver, renal and cardiac function (no history of ischemic heart disease); 4) no prior cancer and/or chemotherapy; and 5) written informed consent. Exclusion criteria included: 1) patients with a history of prior malignancy; 2) pregnant or lactating patients; or 3) without informed consent.
Pretreatment evaluation
Patient pretreatment work-up comprised a complete history, physical examination, full blood count, serum biochemistry, carcinoembryonic antigen measurement, chest radiography, ultrasonography (US) and CT scan of the whole abdomen and pelvis, and colonoscopy with biopsy.
Preoperative chemotherapy
Patients received 4 cycles of a preoperative triplet chemotherapy regimen, which consisted of a 60-min infusion of irinotecan at a dose of 150 mg/m2 on d 1, a 120-min infusion of oxaliplatin at a dose of 85 mg/m2 and a concomitant 120-min infusion of leucovorin at a dose of 200 mg/m2 on d 2, immediately followed by 5-fluorouracil at a dose of 500 mg administered by intravenous bolus, followed by a continuous intravenous infusion of 2,400 mg/m2 over a 44-h period. S-1 (40−60 mg orally twice per day for 10 d) or capecitabine (1,000 mg/m2 orally twice per day for 10 d) could be substituted for 5-fluorouracil in this study. Cycles were repeated every 14 d.
Patients and tumor assessments
Patients were evaluated by CT before treatment, and only patients at clinical stage T4N2M0 (AJCC TNM classification 2009) were included in the study. During the treatment, patients were evaluated by CT every 2 cycles or before the operation. Clinical examinations and complete blood counts were performed every week, and serum biochemistry was performed every two weeks. Toxic side effects were assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC version 4.0).
After neoadjuvant treatment, the primary tumor was re-evaluated with an abdomen and pelvis CT, and the tumor response was assessed by CT tumor volumetry (20,21). The following surgery was scheduled 2 to 6 weeks after completion of neoadjuvant chemotherapy. Surgical management included radical resection of colon cancer with the complete mesocolic excision.
Pathological evaluation of the surgical specimens was performed after the operation. Pathological complete response was defined as the complete disappearance of all cancer cells. Histological regression of the colon tumor was determined according to Mandard tumor regression grading (22,23).
Postoperative chemotherapy
Postoperative adjuvant treatment started within 6 weeks after surgery. It contained 6 cycles of chemotherapy with FOLFOXIRI or XELOX. The substitution of capecitabine with S-1 was allowed.
Statistical analysis
The primary endpoint of the trial was the R0 resection rate, and the secondary endpoints were objective response rate (ORR), time to progression (TTP) and toxicity profile. Statistical analyses were done using the SPSS software (Version 13.0; SPSS Inc., Chicago, IL, USA). The continuous variables were compared by t-test and two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
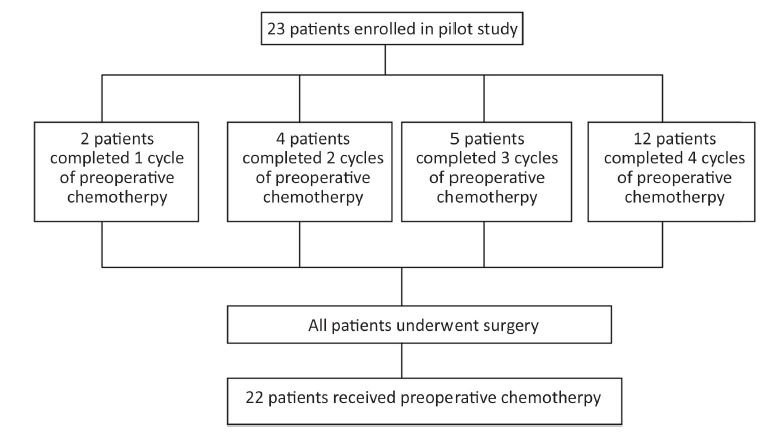
There were 23 patients enrolled in the study from March 2014 to November 2014, and patients' characteristics and demographics are summarized in Table 1. All patients were evaluable for toxicity and response, and they all underwent following operation. The treatment strategy is shown in Figure 1.
Full table
Acute toxicity and treatment compliance
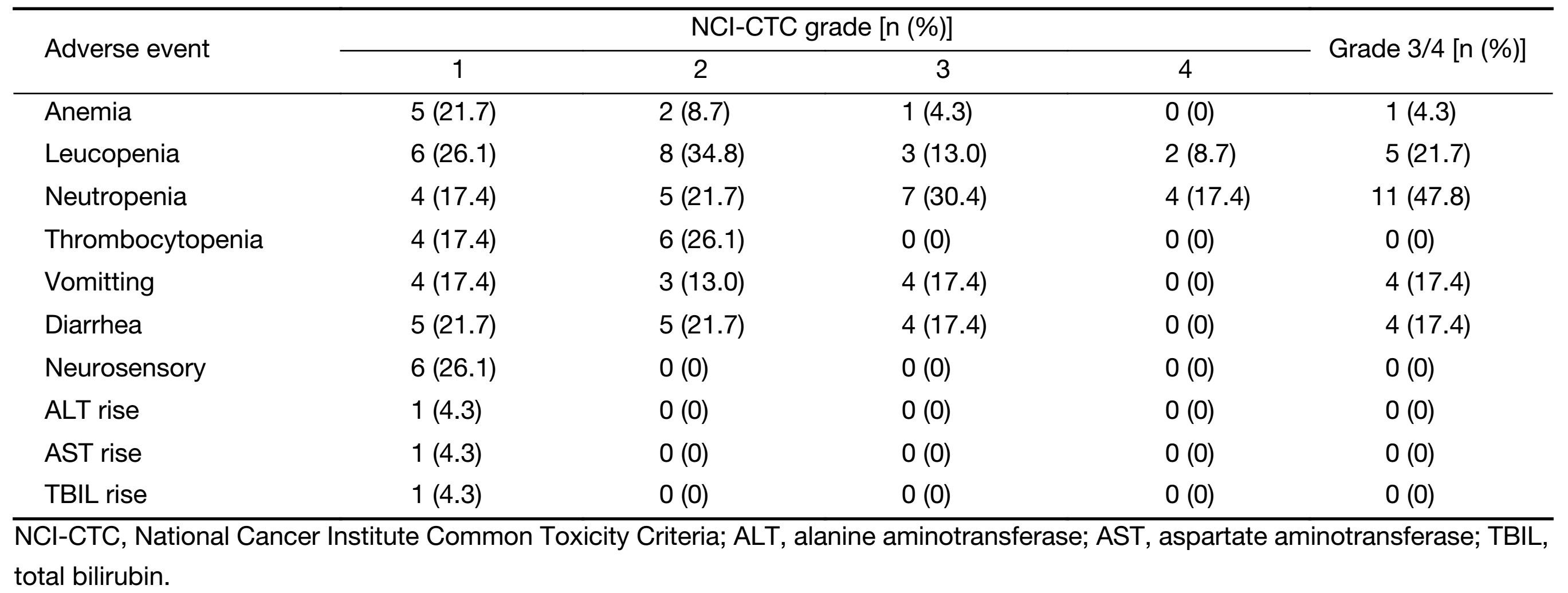
Adverse events (all grades) occurred in all the patients (Table 2), and 13 (56.5%) patients had grade 3/4 toxicity. The most common grade 3/4 toxicities included leucopenia (21.7%), neutropenia (47.8%), vomiting (17.4%) and diarrhea (17.4%). Febrile neutropenia occurred in two (8.7%) patients. Dose reduction was required in 7 (30.4%) patients during preoperative chemotherapy. No treatment-related death occurred.
Full table
Twelve (52.2%) patients completed 4 cycles of neoadjuvant chemotherapy. Among the 23 patients, 4 patients discontinued preoperative treatment because of acute toxicity, one because of colonic obstruction (intussusception confirmed by operation), one because of non-obstructive abdominal pain, and one because of blurred vision, which was later confirmed as retinal artery occlusion, and the other 4 due to non-medical reasons. All the patients subsequently proceeded to surgery.
Evaluation of neoadjuvant chemotherapy efficacy
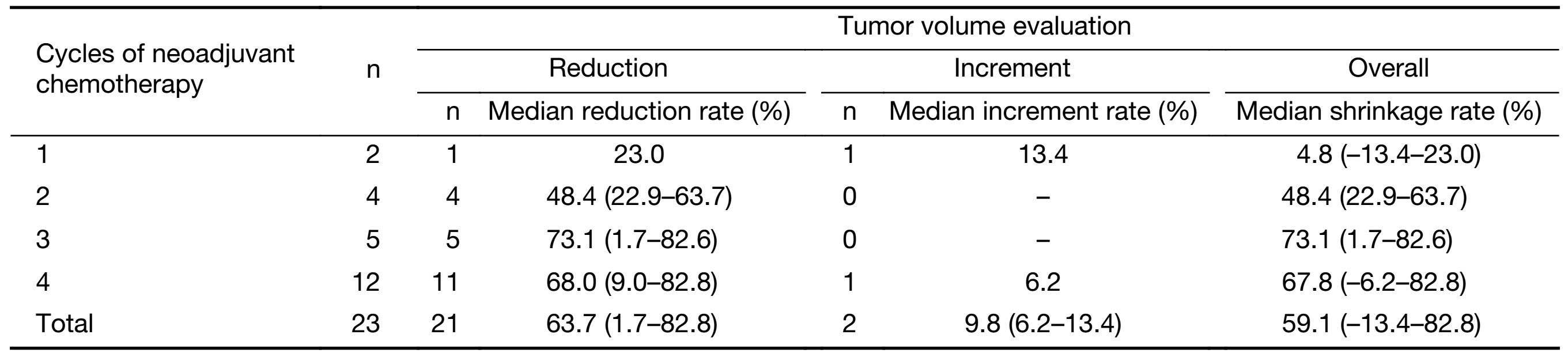
We compared the volume of primary colon cancers measured by CT before and after neoadjuvant chemotherapy. Among the 23 patients, only two patients had increased tumor volumes, and all the others achieved tumor volume reduction (Table 3). The tumor shrinkage rate improved with the increase of neoadjuvant chemotherapy cycles and seemed to be stable after 3 cycles. As a result of small samples, no statistical significant difference could be found between groups of different chemotherapy cycles.
Full table
Surgery and perioperative complications
All patients underwent complete mesocolic excision for colon cancer, among whom 2 patients were found to have pelvic implantation metastasis, and one patient was found to have metastasis in the omentum majus. These 3 patients underwent resection of the primary cancer and implantation metastasis (R1 resection). The other 20 patients underwent radical resection (R0 resection). The operation and perioperative complications are described in Table 4. Patients underwent surgery in 3−45 d (median time: 16 d) after the completion of neoadjuvant chemotherapy. The median operation time was 148 (80−260) min, the median bleeding volume was 30 (20−200) mL, the median time of enteric function restoration was 3 (2−7) d, the median inpatient time after surgery was 9 (7−30) d, and the postoperative inpatient time was all shorter than 12 d except for one patient who had postoperative gastric paralysis for 30 d. All patients had stage I intestinal anastomosis, and only one patient needed a temporary colostomy because of serious intestinal edema.
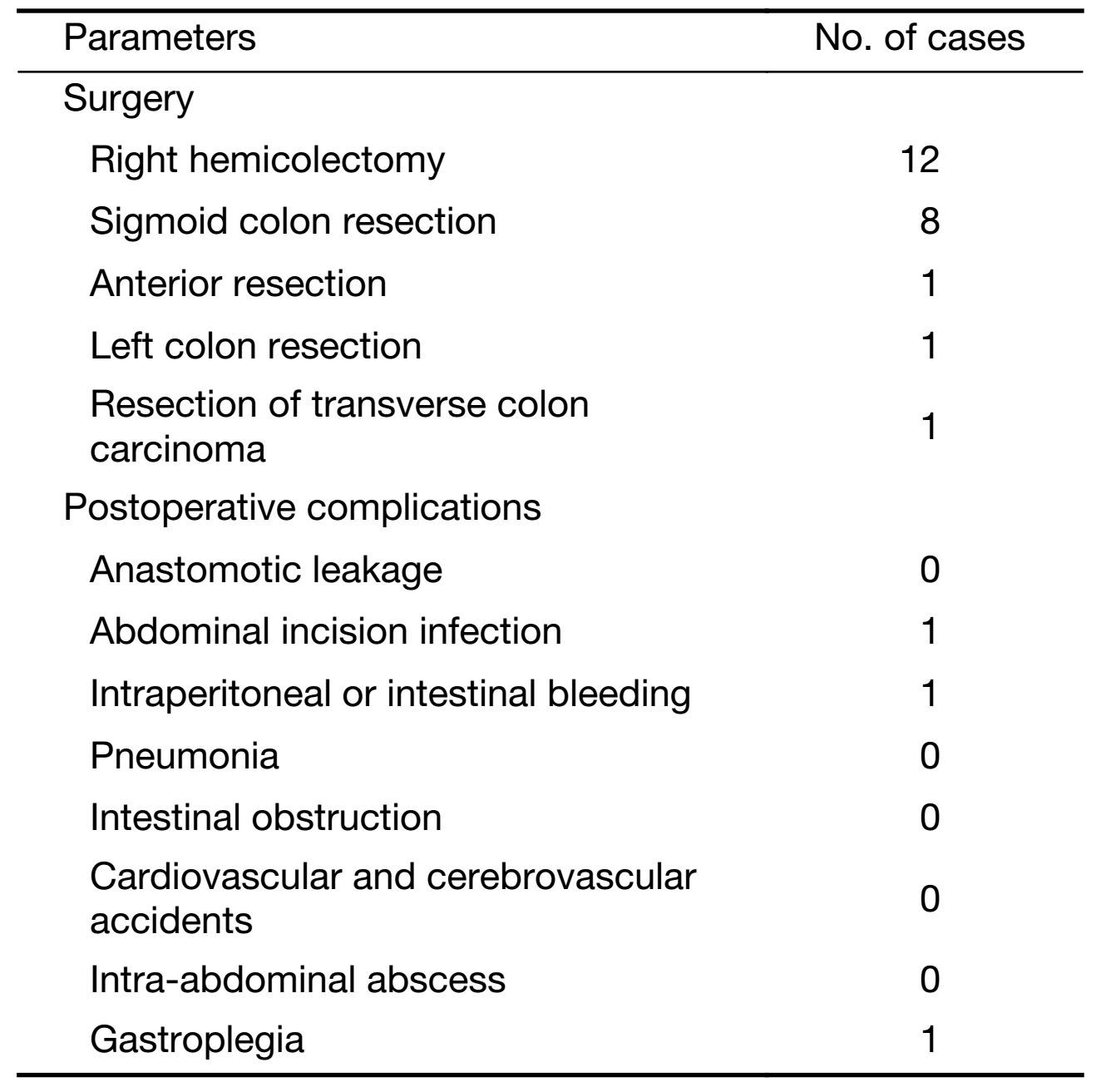
Full table
Efficacy
All surgical specimens underwent pathological examinations, and the pathological TNM staging was compared with preoperative clinical staging (Table 5). Three patients had small peritoneal implantation metastases intraoperatively, and all the visible implantation metastases were contractured and removed completely. All patients had grade I-IV tumor regression, except one patient with grade V regression (Table 6).
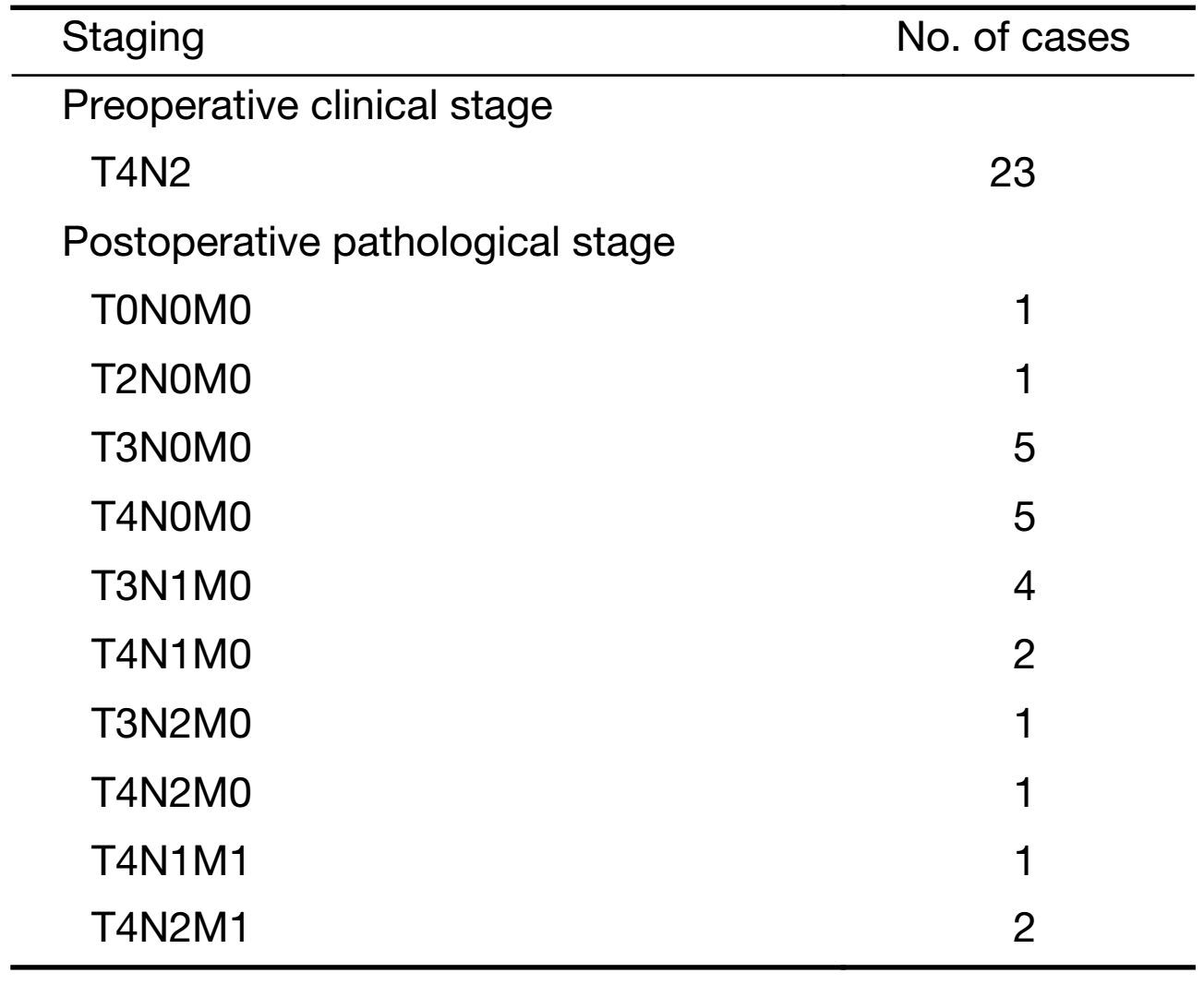
Full table

Full table
Among T3N1M0 patients, one patient had 10 lymph nodes with a mucous lake, but no cancer cells remained. Furthermore, three T3−4N0M0 patients had a mucous lake only in some lymph nodes.
Postoperative chemotherapy
Twenty-two patients received postoperative adjuvant chemotherapy. The median time from surgery to initiation of adjuvant chemotherapy was 34 d (18−109 d). Eight patients were treated with the FOLFOXIRI regimen, 12 patients received oxaliplalin combined with capecitabine or S-1, and the other 2 patients received off-protocol treatments (1 patient received irinotecan plus S-1, the other patient received irinotecan, S-1 and bevacizumab). Ten patients (43.5%) completed at least 6 cycles of postoperative chemotherapy, and the median number of cycles of postoperative chemotherapy for all the patients was 5. Grade 3/4 adverse events were uncommon, and they included leucopenia (2/23), neutropenia (3/23), febrile neutropenia (1/23), thrombocytopenia (1/23) and diarrhea (3/23). No treatment-related death occurred.
Follow-up
At present, all patients have completed the adjuvant chemotherapy and the follow-up with a median time of 28 (24–32) months which is counted from the date of initial neoadjuvant chemotherapy. Six patients developed disease progression, including one patient with lung metastases, three with liver metastases, one with metastasis in the bilateral adnexa and one with peritoneal metastasis. Among the six patients, one patient died of cancer recurrence. The 2-year OS rate is 95.7% (22/23), and the recurrence-free survival rate as well the DFS rate are both 73.9% (17/23). The 2-year recurrence rate is 26.1% (6/23).
Discussion
Presently, some prospective neoadjuvant chemotherapy clinical studies on metastatic colon cancer have been carried out. The FOLFOXIRI regimen has become one of the first-line chemotherapeutic treatments for its high efficacy, although its toxicity is severe (19-21). However, for locally advanced resectable colon cancer, there has been only one prospective randomized controlled clinical study internationally, the FOxTROT study (14). The study showed that preoperative chemotherapy combining two chemotherapeutic agents (oxaliplatin and 5-fluorouracil) had a significant effect on tumor down-staging and could be used safely in colon cancer patients who did not have distant metastasis.
We designed this study to investigate whether the FOLFOXIRI regimen, which combined three chemotherapeutic agents and had high efficacy, could be used in patients with locally advanced resectable colon cancer. We recruited patients with severe locally advanced disease of clinical stage T4N2M0 to reduce excessive chemotherapy treatments, but we actually found that three patients had intraperitoneal implantation metastasis. Thus, these results showed that preoperative staging might underestimate tumor stage for patients with locally advanced resectable colon cancer. The FOLFOXIRI regimen combining with targeted therapy was not included in this study, considering that the targeted therapy had not been shown to improve adjuvant therapy for patients with stage III colon cancer.
Our results showed that tumor volume markedly decreased in most patients, even in patients who received only one cycle of neoadjuvant chemotherapy. And for the patients who completed 3–4 cycles, the tumor down-staging was more obvious. In the study, only two patients had tumor progression, but they all underwent R0 resection.
The rate of grade 3–4 toxicities was up to 56.5% in all patients because the FOLFOXIRI regimen, which combined three chemotherapeutic agents, had high toxicity. However, the toxicity did not affect the following surgery. All patients received surgery within a month after chemotherapy with a median time of 16 d, except one patient who received surgery on the 45th d after chemotherapy because of sustained bone marrow suppression. During the course of preoperative chemotherapy, there was only one emergency operation, which was acute intestinal obstruction caused by intussusception. Meanwhile, chemotherapy toxicity did not result in greater surgical complications. All 23 patients underwent tumor resection and intestinal anastomosis, and no patient had severe complications, such as anastomotic leakage, bleeding or reoperation. Only one patient underwent temporary colostomy due to bowel edema. Only three patients suffered from wound infection, gastric paralysis and intestinal bleeding. These symptoms were all relieved after corresponding treatment. Thus, we believe that the toxic side effects of neoadjuvant chemotherapy with the FOLFOXIRI regimen and the effects on the operations are totally acceptable.
In agreement with the high tumor shrinkage rate of preoperative chemotherapy, postoperative pathology also showed significant tumor down-staging, although clinical and pathological staging were not entirely consistent. We believe that further randomized controlled studies can be more accurate in revealing the effects of the FOLFOXIRI regimen on tumor down-staging and the effects of neoadjuvant chemotherapy on the survival of patients with resectable colon cancer in the future.
Conclusions
Although the FOLFOXIRI regimen combining three chemotherapeutic agents has high toxicity, the regimen has remarkable efficacy, and the side effects are tolerable for patients with locally advanced resectable colon cancer. At the same time, preoperative chemotherapy with FOLFOXIRI seems to cause no serious complications after surgery. Thus, we believe that FOLFOXIRI can be safely and effectively used in patients with locally advanced resectable colon cancer in neoadjuvant chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [PubMed] DOI:10.3322/caac.v66.4
- Liu S, Zheng R, Zhang M, et al. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res 2015;27:22–8. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.02.01
- Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 1988;80:30–6. [PubMed] DOI:10.1093/jnci/80.1.30
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [PubMed] DOI:10.1200/JCO.2008.20.6771
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204. [PubMed] DOI:10.1200/JCO.2006.08.2974
- Newton AD, Datta J, Loaiza-Bonilla A, et al. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol 2015;6:534–43. [PubMed] DOI:10.3978/j.issn.2078-6891.2015.047
- Graham PJ, Brar MS, Foster T, et al. Neoadjuvant chemotherapy for breast cancer, is practice changing? A population-based review of current surgical trends. Ann Surg Oncol 2015;22:3376–82. [PubMed] DOI:10.1245/s10434-015-4714-x
- Gotto GT, Shea-Budgell MA, Rose MS, et al. Predictors of referral for neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer and changes in practice over time. Can Urol Assoc J 2015;9:236–41. [PubMed] DOI:10.5489/cuaj.2722
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74. [PubMed] DOI:10.1200/JCO.2011.36.4539
- Robinson S, Manas DM, Pedley I, et al. Systemic chemotherapy and its implications for resection of colorectal liver metastasis. Surg Oncol 2011;20:57–72. [PubMed] DOI:10.1016/j.suronc.2009.10.002
- Nordlinger B, Van Cutsem E, Gruenberger T, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 2009;20:985–92. [PubMed] DOI:10.1093/annonc/mdn735
- Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 2008;26:1830–5. [PubMed] DOI:10.1200/JCO.2007.13.7679
- Robinson SM, Wilson CH, Burt AD, et al. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol 2012;19:4287–99. [PubMed] DOI:10.1245/s10434-012-2438-8
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152–60. [PubMed] DOI:10.1016/S1470-2045(12)70348-0
- Falcone A, Masi G, Allegrini G, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J Clin Oncol 2002;20:4006–14. [PubMed] DOI:10.1200/JCO.2002.12.075
- Masi G, Allegrini G, Cupini S, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of phase II study with a simplified biweekly schedule. Ann Oncol 2004;15:1766–72. [PubMed] DOI:10.1093/annonc/mdh470
- Masi G, Cupini S, Marcucci L, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 2006;13:58–65. [PubMed] DOI:10.1245/ASO.2006.03.094
- Souglakos J, Mavroudis D, Kakolyris S, et al. Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol 2002;20:2651–7. [PubMed] DOI:10.1200/JCO.2002.08.015
- Montagnani F, Chiriatti A, Turrisi G, et al. A systematic review of FOLFOXIRI chemotherapy for the first-line treatment of metastatic colorectal cancer: improved efficacy at the cost of increased toxicity. Colorectal Dis 2011;13:846–52. [PubMed] DOI:10.1111/codi.2011.13.issue-8
- Murono K, Kawai K, Tsuno NH, et al. Barium enema and CT volumetry for predicting pathologic response to preoperative chemoradiotherapy in rectal cancer patients. Dis Colon Rectum 2014;57:715–24. [PubMed] DOI:10.1097/DCR.0000000000000070
- Arredondo J, González I, Baixauli J, et al. Tumor response assessment in locally advanced colon cancer after neoadjuvant chemotherapy. J Gastrointest Oncol 2014;5:104–11. [PubMed] DOI:10.3978/j.issn.2078-6891.2014.006
- Suárez J, Vera R, Balén E, et al. Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis 2008;10:563–8. [PubMed] DOI:10.1111/j.1463-1318.2007.01424.x
- Santos MD, Silva C, Rocha A, et al. Prognostic value of mandard and dworak tumor regression grading in rectal cancer: study of a single tertiary center. ISRN Surg 2014;2014:310542. [PubMed] DOI:10.1155/2014/310542