Boron neutron capture therapy for malignant melanoma: first clinical case report in China
Introduction
Boron neutron capture therapy (BNCT) is a promising cancer treatment which exploits the neutron capture and fission reaction of the boron isotope, 10B. When the nonradioactive 10B in cells captures low-energy thermal neutrons, it can produce high linear energy transfer (LET) α particles and lithium isotope 7Li nuclei that are lethal to these cells. The noteworthy point is that this destructive effect is confined to boron-containing cells, as the path lengths of LET particles are 5−9 μm, almost the size of a cell. Consequently and theoretically, malignant tumor cells can be selectively destroyed if they absorb more 10B than normal cells when a 10B-containing drug is delivered into the body (1,2).
In this way, BNCT provides a solution to the following two problems. Firstly, radiation-related damage could be greatly decreased as BNCT has the potential to destroy tumor cells selectively and spare normal cells. Patients could not only enjoy a much better quality of life, but also be more tolerant of radiotherapy and benefit from this treatment. Secondly, BNCT has the potential to manage some tumors not successfully treated by current therapies, such as high-grade gliomas, head and neck cancers, and malignant melanoma (3-7).
However, at least two factors have dramatically restricted the therapeutic value of BNCT. On the one hand, no agent at this time meets the requirements of a successful boron delivery agent (8). The only two United States Food and Drug Administration-approved boron drugs are boronophenylalanine (BPA) and sodium borocaptate (BSH). They have been used for more than 50 years. However, they are nonselective and not ideal for BNCT. On the other hand, a lack of an in-hospital neutron irradiator (IHNI) has for a long time limited BNCT as an experimental treatment. Previously, studies were all carried out in nuclear research reactors. They were often distant from medical facilities and were not suitable for medical purposes. The sad truth was that they were the only available neutron source at the time, and what is worse, many of them are now either closed or under the threat of closure (1).
Undoubtedly, the invention and construction of an IHNI is an important step for BNCT to progress from the experimental stage to formal clinical practice. In 2010, the construction of the world's first IHNI was completed in Beijing, China (9,10). We initiated this first clinical trial to investigate whether the IHNI had sufficient potential to serve as a neutron source within a hospital for BNCT, and we report here the results of the first patient treated by an IHNI according to the protocol of BNCT.
Study protocol
This clinical trial was approved by the Medical Ethics Committee of the Third Xiangya Hospital of Central South University, Changsha, China, which was fully accredited by the Association for the Accreditation of Human Research Protection Program (AAHRPP) and conformed to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). In addition, the trial was conducted in accordance with the protocol as per ClinicalTrials.gov (No. NCT02759536).
The inclusion criteria included: 1) signed informed consent form; 2) age ≥18 years; either gender; 3) life expectancy ≥3 months; 4) features of disease: i) tissue biopsy and pathological analysis confirm the diagnosis of melanoma; ii) positron emission tomography-computed tomography (PET/CT) and serum lactate dehydrogenase (LDH) can be used for auxiliary diagnosis; iii) diameter of at least one solid tumor ≥1 cm; 5) Karnofsky performance status (KPS) score ≥70%; 6) Eastern Cooperative Oncology Group (ECOG) score: 0−2 grade; 7) within a week, complete blood count: hemoglobin ≥90 g/L, leukocyte ≥4.0×109/L, neutrophilic granulocyte ≥2.0×109/L, platelet ≥100.0×109/L; and renal function: creatinine ≤180 μmol/L; 8) never accepted radiation or chemotherapy, or the interval of radiation or chemotherapy in the last 3 months; 9) never accepted target drugs or biotherapeutics in the last 3 months; and 10) urine pregnancy test negative.
The exclusion criteria included: 1) intolerable to BNCT treatment; 2) severe coagulation disorders; 3) poor compliance; 4) severe complications or infection without control; 5) pregnant or lactating women; 6) patients with metallic instruments (e.g. pacemaker, artificial limb); 7) boron concentration in tumor tissue <1.5 times that in blood based on boron pharmacokinetics study (boron concentration ratio of tumor tissue to blood sample (T/B) <1.5); or 8) age <18 years.
Case report
Case presentation
A male patient (taxi driver) presented to the Department of Dermatology of the Third Xiangya Hospital of Central South University, Changsha, China, in August 2014 complaining of pigmented lesions on the sole of his left foot. A lesion was first noticed in the plantar site of his left foot 7 years previously and it grew wider gradually. It received no treatment at the time. Another lesion was initially noticed 2 years prior in the heel of the same foot, and had expanded quickly over the preceding 6 months. In addition, the patient complained of itching and pain in the heel. The patient underwent laparoscopic cholecystectomy in 2004, but no other significant information was obtained from his family history or personal history.
Physical examination showed that both lesions were asymmetric in shape, with irregular borders and color variegation. An ulcer was noticed on the lesion in the heel, and that area was moderately raised with sensitive tenderness. No inguinal lymph nodes or popliteal lymph nodes were palpable, and there were no other similar skin lesions in other parts of the body (Figure 1A).
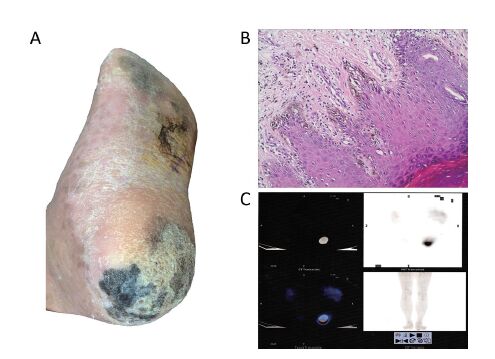
Based on the presentation, a clinical diagnosis of malignant melanoma was made. A punch biopsy was performed for histopathological analysis. It was reported that intermittent clusters of nevus cells were observed in the stratum basale, and these cells were proliferating actively, suggesting superficial spreading malignant melanoma in situ (Figure 1B). A PET/CT scan revealed abnormal fluorodeoxyglucose (FDG) uptake in the heel of the left foot, indicating malignant melanoma (Figure 1C). Besides, no metastasis was revealed by PET/CT scan or magnetic resonance imaging (MRI).
On the basis of his disease history, physical examination and imaging examination, surgery was recommended as his first-line treatment and left foot amputation was advised. However, the patient rejected this option, and demanded to be enrolled as the first patient after hearing about the IHNI clinical trial project.
Treatment procedure and results
Boron pharmacokinetic profile
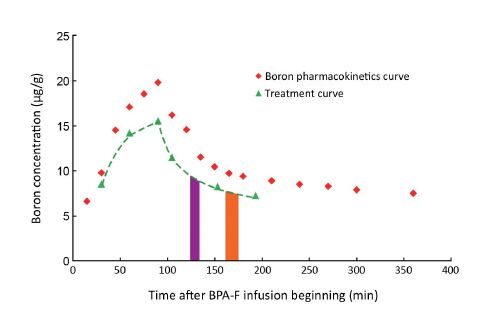
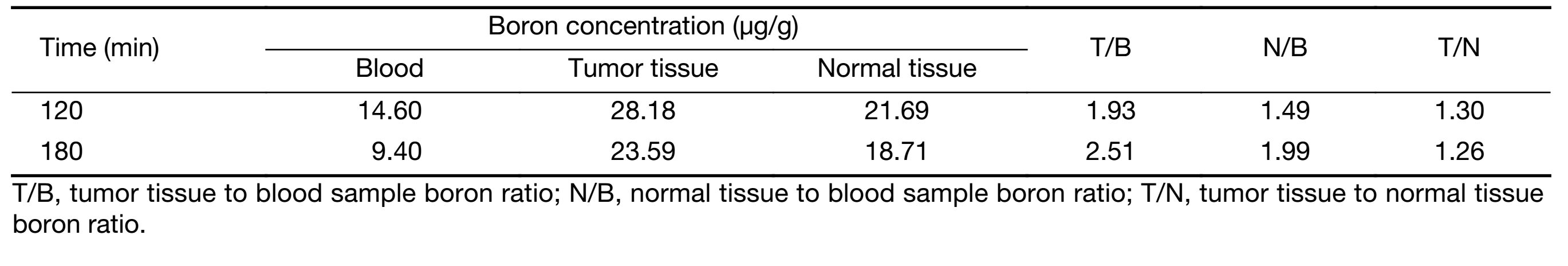
A BPA-fructose (BPA-F) complex was used as the boron delivery agent. To calculate the pre-planning dosimetry, a pharmacokinetic study of BPA was performed in the Third Xiangya Hospital on August 12, 2014. According to the protocol, BPA-F at a dose of 350 mg/kg was infused into the patient intravenously over 90 min. Before BPA-F infusion, 50 mL blood sample was taken, and 15 mL blood samples were taken every 15 min in the following 180 min after BPA-F infusion began. Then, 10−15 mL blood samples were taken every 30 min till 300 min after BPA-F infusion began. At 120 min and 180 min after the beginning of BPA-F infusion, tumor tissues and normal tissues (from the left foot and determined by two dermatologists) were obtained from the patient. The concentration of 10B in all blood samples and tissues was measured by inductively coupled plasma atomic emission spectrometry (ICP-AES). Boron concentration values in blood samples versus time were plotted and the boron concentration ratios of tumor tissue to blood sample (T/B) and normal tissue to blood sample (N/B) at these two time points were calculated (Figure 2,Table 1).
Full table
Patient positioning
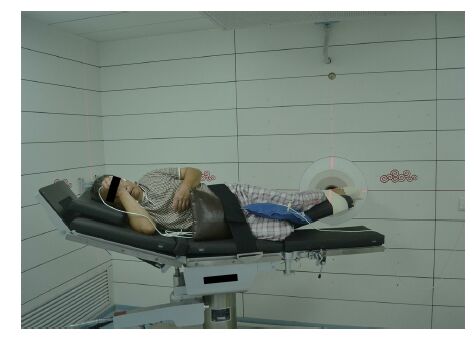
Patient positioning was carried out in the simulation room (SR) of the IHNI. SR replicated the geometry of the irradiation room (IR) of the IHNI and employed a plastic model to mimic the beam outlet of the IR. Then the optimal patient positioning was determined and fixed by means of positioning devices. Laser collimators on the walls of the SR helped the marking of reference points on the patient's foot which would be used for accurate repositioning in the IR (Figure 3).
Dose calculation and patient irradiation
The diameter of the beam outlet of IR was 14 cm. To guarantee that the lesions on the patient's left foot received effective irradiation, two fields were planned. The first field was planned to cover the heel, and the second, the plantar site.
The patient was planned for BNCT on August 19, 2014. On that day, a 50 mL blood sample was obtained and then the patient received BPA-F infusion at 9:00 AM at a dose of 350 mg/kg over 90 min. During this time period, 10−15 mL blood samples were taken every 30 min. Another three blood samples (10−15 mL) were obtained 105 min, 150 min and 180 min after the BPA-F infusion began. The boron concentration of these samples was measured immediately and blood concentration values versus time were plotted (treatment curve), comparing the boron pharmacokinetics curve (Figure 2). When the time was taken into account for measuring the boron concentration of samples and for repositioning of the patient in the IR, the irradiation of the first and the second fields was determined to begin at 123 min and at 161 min respectively after the start of BPA infusion. Skin was considered as the organ that limits the maximum radiation dose, and according to the studies by González et al. (11) and Fukuda et al. (12), the maximum dose of skin was 16.5 relative biological effectiveness (RBE) Gy and a minimum dose for tumor control was 20 RBE Gy. The irradiation time was calculated by the Monte Carlo N Particle Transport Code 6 (MCNP 6) program, based on the maximum radiation dose of skin, thermal neutron beam flux of IHNI, boron concentration in blood, and ratio value of T/B and N/B measured during the boron pharmacokinetics test. The irradiation time of the first field was calculated to be 10.5 min and the second field was 12 min (Figure 2).
Clinical outcome evaluation
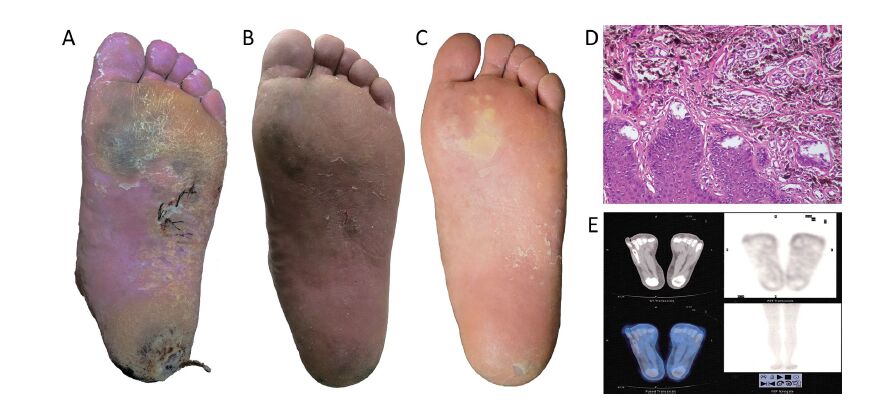
After 1 week from thermal neutron irradiation, no cardinal signs of inflammation were detected on the patient's left foot. Only slight dandruff was observed on the surface of the irradiated area. Blood tests and urine analysis at this time showed no significant findings. According to the Acute Radiation Morbidity Scoring Criteria and Late Radiation Morbidity Scoring Scheme from the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC), grade 1 acute radiation injury was determined. During the following 4 weeks, exfoliation was noticed in the sole of the patient's left foot (Figure 4A). At this time, grade 2 acute radiation injury was determined. Lipido-colloid dressing (Urgotul® Duo, Urgo Medical, France) and an alginate wound dressing (SeaSorb®, Coloplast A/S, Denmark) were then applied on the plantar site and heel respectively. After 5 weeks from irradiation, dandruff was no longer observed on the irradiated area, and the results of the patient's blood tests were normal. After 6 months from BNCT treatment, the lesions in the sole of the patient's left foot had shrunk greatly (Figure 4B), and on August 19, 2016 (24 months after irradiation), these lesions had further diminished substantially (Figure 4C). To rule out the possibility of remnant cancer in these areas, another biopsy was performed in both skin lesions for pathological analysis on May 19, 2015, 9 months after BNCT. Epidermal hyperplasia and many spindle-like pigment-containing cells in the superficial layer of the dermis were observed. These pigment-containing cells did not form a nest or invade into the deep layer of dermis (Figure 4D), and PET/CT scan performed 24 months after BNCT showed no abnormal FDG uptake in his left foot nor any other parts of his body (Figure 4E). In addition, no late radiation injury was observed during the 24-month follow-up.
Discussion
Malignant melanoma is a lethal skin cancer whose incidence has continued to increase in many countries (13,14). Since it has a poor sensitivity to radiotherapy and no ideal chemotherapy protocol works well on this disease, wide surgical excision has been recommended as the first-line treatment for this disease, especially in the early stages (15). In Asian countries like China, the most frequent subtype of melanoma is acral melanoma. However, the 5- and 10-year disease-specific survival rates of this subtype after surgery in China were 53.3% and 27.4%, according to a recent study (16). The prognosis of the disease at advanced stages was even worse, as the median survival time was 6−8 months and the 5-year survival rate was about 6% with chemotherapy (17).
BNCT might change this phenomenon. In a study carried out by Fukuda et al., 16 of 22 patients who received BNCT showed a complete response (12). In 2004, the first case of malignant melanoma treated by BNCT was reported in Argentina, and 21 of the 25 treated subcutaneous melanoma nodules showed complete response 8 weeks after the treatment (11). Although these studies were not randomized controlled trials and some of them were case reports, they did suggest that BNCT could be a promising treatment for malignant melanoma.
In our study, this patient was the first case treated by BNCT in China, in a neutron irradiator which was specifically designed for medical applications. The patient was followed up for 24 months. During this period, the pigmented lesions in his left foot diminished considerably, leaving a few small pigmented patches which most likely consisted of dead pigment-containing cells, since pathological analysis performed 9 months after irradiation suggested that these pigment-containing cells did not form a nest nor invaded the deep layer of dermis. Further, PET/CT examination 24 months after irradiation revealed no lesions in his foot, or metastases in any other place in his body. The patient's quality of life was improved greatly as the ulcer had healed and there was no pain or itching in his left foot. As mentioned above, surgery is the recommended therapy for malignant melanoma (15). However, as we can see from this case, surgery may act as a double-edged sword for patients. The surgical removal of the patient's foot would have led to disability further impacting his occupation and quality of life. BNCT might be a better therapy for achieving the same goal. Consequently, the outcome of this study also prompted us to think that we might be too conservative in demonstrating the value and potential of BNCT in treating tumors, since BNCT was predominantly applied in tumors at an advanced stage in previous studies. In particular, when future efforts lead to the invention of new boron delivery drugs that can achieve much higher tumor tissue to normal tissue (T/N) boron ratios or are retained in tumor tissues for a longer time, this would provide a greater potential for BNCT to destroy malignant cells more precisely.
The outcome of this case also demonstrated that the IHNI has potential for the delivery of BNCT, even though its thermal power was only 30 kW and was much lower than that of current nuclear reactors. Until now, no randomized controlled trials of BNCT were carried out in the world, in a large part due to the fact that the nuclear research reactors in use were of different power and difficult to manage, and the protocols and treatment plans used in different research groups varied considerably. This situation might be changed by the IHNI as it can be built in many hospitals and be operated easily by medical staff. It would not be radical to expect that a multi-centered, randomized controlled trial of BNCT would become possible in the near future.
Acknowledgements
We thank Daqing Cui for assistance in conducting this clinical trial.
Funding: This work was supported by the National Science & Technology Pillar Program during the 12th Five-Year Plan Period (No. 2013BAI01B08) and the Major Program of the National Natural Science Foundation of China (No. 51290295).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barth RF, Coderre JA, Vicente MG, et al. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 2005;11:3987–4002. [PubMed] DOI:10.1158/1078-0432.CCR-05-0035
- Mirzaei HR, Sahebkar A, Salehi R, et al. Boron neutron capture therapy: Moving toward targeted cancer therapy. J Cancer Res Ther 2016;12:520–5. [PubMed] DOI:10.4103/0973-1482.176167
- Wang LW, Chen YW, Ho CY, et al. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: a prospective phase I/II trial. Int J Radiat Oncol Biol Phys 2016;95:396–403. [PubMed] DOI:10.1016/j.ijrobp.2016.02.028
- Miyatake S, Kawabata S, Hiramatsu R, et al. Boron neutron capture therapy for malignant brain tumors. Neurol Med Chir (Tokyo) 2016;56:361–71. [PubMed] DOI:10.2176/nmc.ra.2015-0297
- Liu YW, Chang CT, Yeh LY, et al. BNCT treatment planning for superficial and deep-seated tumors: Experience from clinical trial of recurrent head and neck cancer at THOR. Appl Radiat Isot 2015;106:121–4. [PubMed] DOI:10.1016/j.apradiso.2015.08.002
- Lim D, Quah DS, Leech M, et al. Clinical potential of boron neutron capture therapy for locally recurrent inoperable previously irradiated head and neck cancer. Appl Radiat Isot 2015;106:237–41. [PubMed] DOI:10.1016/j.apradiso.2015.07.044
- Menéndez PR, Roth BM, Pereira MD, et al. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot 2009;67:S50–3. [PubMed] DOI:10.1016/j.apradiso.2009.03.020
- Moss RL. Critical review, with an optimistic outlook, on boron neutron capture therapy (BNCT). Appl Radiat Isot 2014;88:2–11. [PubMed] DOI:10.1016/j.apradiso.2013.11.109
- Ke G, Sun Z, Shen F, et al. The study of physics and thermal characteristics for in-hospital neutron irradiator (IHNI). Appl Radiat Isot 2009;67:S234–7. [PubMed] DOI:10.1016/j.apradiso.2009.03.117
- Zhang Z, Chong Y, Chen X, et al. PGNAA system preliminary design and measurement of in-hospital neutron irradiator for boron concentration measurement. Appl Radiat Isot 2015;106:161–5. [PubMed] DOI:10.1016/j.apradiso.2015.07.049
- González SJ, Bonomi, M R, Santa Cruz GA, et al. First BNCT treatment of a skin melanoma in Argentina: dosimetric analysis and clinical outcome. Appl Radiat Isot 2004;61:1101–5. [PubMed] DOI:10.1016/j.apradiso.2004.05.060
- Fukuda H, Hiratsuka J, Kobayashi T, et al. Boron neutron capture therapy (BNCT) for malignant melanoma with special reference to absorbed doses to the normal skin and tumor. Australas Phys Eng Sci Med 2003;26:97–103. [PubMed] DOI:10.1007/BF03178777
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [PubMed] DOI:10.3322/caac.21254
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [PubMed] DOI:10.3322/caac.21338
- Schadendorf D, Fisher DE, Garbe C, et al. Melanoma. Nat Rev Dis Primers 2015;1:15003. [PubMed] DOI:10.1038/nrdp.2015.3
- Lv J, Dai B, Kong Y, et al. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep 2016;6:31432. [PubMed] DOI:10.1038/srep31432
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–34. [PubMed] DOI:10.1200/jco.2001.19.16.3622
