Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis
Introduction
Although the incidence of gastric cancer has decreased over the years, it is the fifth leading cause of cancer worldwide after lung, breast, colorectal and prostate cancers and it is the third most common cause of cancer deaths worldwide after lung and liver cancers (1,2). Gastric cancer accounted for 10% of the total cancer-related deaths and 8% of the total cancer cases in 2008 with over 70% of new cases and deaths occurring in developing countries (3). Asia and Eastern Europe have the highest rates of disease (4). The 5-year overall survival (OS) for gastric cancer is approximately 15%−20% and the survival rate drops steeply as the staging progresses. When localized to the stomach the 5-year OS is approximately 55%, but by stage IV the 5-year OS decreases to 4%.
Peritoneal carcinomatosis arising from gastric cancer is generally associated with poor prognosis. Risk factors found to be significantly associated with peritoneal carcinomatosis in literature include tumor stage T3/T4 (5-9), age ≤60 years (9), histological type (including signet-ring cell features) (6,10), nodal invasion (7,8), vascular invasion (6), ascites (5), liver metastasis (5), and female gender (10). The median survival rates for peritoneal carcinomatosis range from 1 to 9 months with no survival at the 5th year (11).
Recently, Thomassen et al. (10) did a population-based study to investigate the morbidity and mortality among patients with peritoneal carcinomatosis of gastric origin and found the median survival of patients with peritoneal metastasis to be 4 months as compared to 14 months in patients without metastasis. Out of the 5,220 patients studied, 39% of them presented with metastatic disease and 35% of them had peritoneal carcinomatosis. Similarly, Sadeghi et al. (5) found the OS was 3.1 months and over half were diagnosed with peritoneal carcinomatosis at the time of primary gastric cancer diagnosis in their prospective trial.
Surgery along with adjuvant chemotherapy or chemoradiation has been the mainstay treatment of non-metastatic gastric cancer over the years with palliative systemic chemotherapy being the standard of care in advanced or recurrent gastric cancer. Within the last three decades, there has been an increasing interest in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in treating advanced gastric carcinoma with peritoneal carcinomatosis with the goal of killing any residual microscopic disease that may be present after completely removing the macroscopic disease. The intent of this article is to review literature dealing with treatments for gastric cancer with peritoneal carcinomatosis including chemotherapy, surgery, and HIPEC.
Current approaches for gastric cancer with peritoneal carcinomatosis
Chemotherapy and surgery
Palliative chemotherapy is the standard of care in advanced or recurrent gastric cancer. Wagner et al. (12) published a systematic review in 2010 that evaluated the effect of chemotherapy in patients with advanced gastric cancer as compared to best supportive care. They found a 6.7 month improvement in median survival (from 4.3 to 11.0 months) in the chemotherapy group when compared with the best supportive care (Table 1). In 2013, Global Advanced/Adjuvant Stomach Tumor Research International Collaboration (GASTRIC) (13) did a meta-analysis on the efficacy of chemotherapy on OS and progression-free survival (PFS) in advanced/recurrent gastric cancer. When comparing the experimental chemotherapy arms with the corresponding control arms, the hazard ratio was 0.88 and 0.81 in regards to OS and PFS. These hazard ratios equated to a median OS difference of 3 weeks (37.6 and 34.4 median in weeks) and a PFS difference of 4 weeks (20.4 and 16.4 median in weeks). The GASTRIC study exemplified how chemotherapy in addition to standard regimens has yielded minimal improvement in OS and progression-free intervals with no certain chemotherapy regimen emerging as a better standard (13). Although chemotherapy is the mainstay treatment for advanced gastric cancer, there does not seem to be any set consensus on the overall efficacy of treatment.
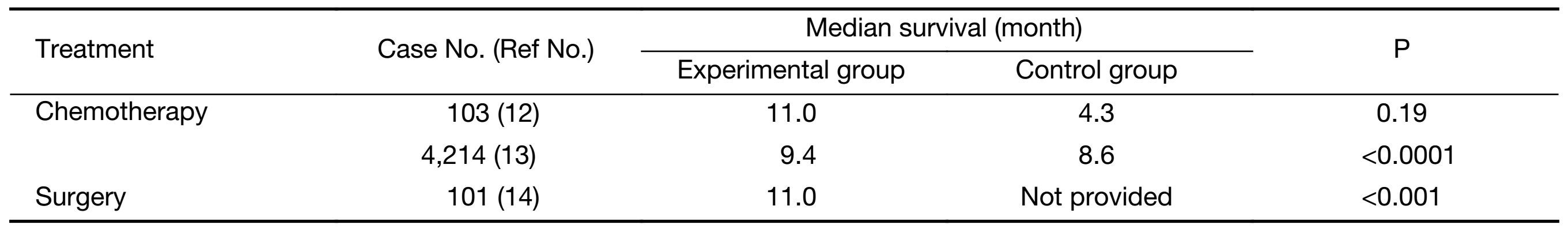
Full table
The outcome of surgical intervention is often predicated by the progression of gastric cancer. Hioki et al. (14) found that gastrectomy increased survival in patients with metastasis to adjacent peritoneum (P1) and few scattered metastasis to adjacent peritoneum (P2) but not in patients with numerous distant peritoneal metastasis (P3). They found that the OS in P1, P2 and P3 groups was 18 months, 15 months and 9 months, respectively. Furthermore, the 1-year survival was found to be 64.7%, 69.2% and 35.2%, respectively. However, Kim et al. (9) found that there was no significant trend of improved survival after surgical management of HIPEC.
Currently, there are no large randomized studies examining the efficacy of surgery and systemic therapy versus systemic therapy alone in patients with advanced metastatic gastric cancer. The GYMSSA trial will be conducted by the National Cancer Institute and will compare gastrectomy, metastectomy plus systemic therapy versus systemic chemotherapy alone in metastatic gastric cancer patients (15). This trial will highlight whether or not there is a benefit to aggressive surgical intervention in addition to the current chemotherapeutic standard of care.
CRS plus HIPEC versus chemotherapy
Hultman et al. (16) compared systemic chemotherapy followed by CRS and HIPEC with systemic chemotherapy only in patients with peritoneal carcinomatosis from gastric cancer and found the mean OS in the experimental group was 20.5 months as compared to 11.1 months in the control group (Table 2).
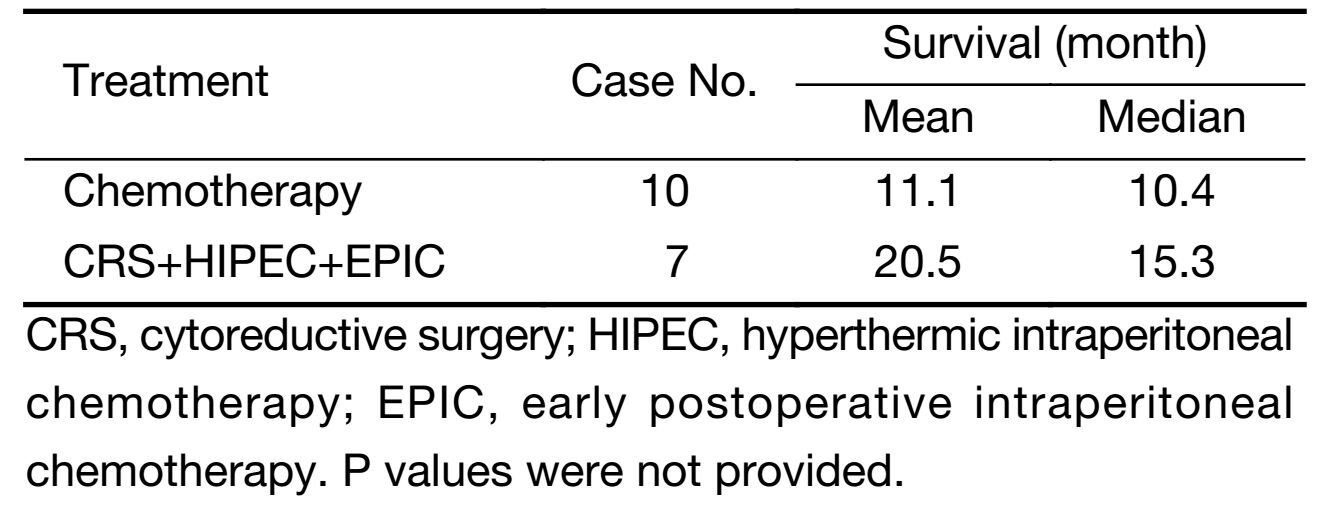
Full table
CRS plus HIPEC versus surgery
It has been shown that if intraperitoneal free cancer cells are present after curative surgery in advanced gastric patients, the 5-year survival rate is only 15.4%, as compared to 49.4% if no intraperitoneal cancer cells are found (17). Furthermore, Kuramoto et al. (18) found that extensive intraoperative peritoneal lavage followed by intraperitoneal chemotherapy (EIPL-IPC) significantly increased the 5-year survival rate on patients with intraperitoneal free cancer cells without overt peritoneal metastasis (CY+/P) as compared to surgery plus IPC and surgery alone groups. The 5-year survival rate was 43.8%, 4.6% and 0% in the EIPL-IPC, IPC and surgery alone groups, respectively (18). The goal of HIPEC in killing off microscopic tumor cells that may be present after CRS is a promising treatment modality for gastric cancer with peritoneal carcinomatosis.
There are a limited amount of randomized trials on CRS and HIPEC in gastric cancer with peritoneal carcinomatosis. Yang et al. (19) completed a randomized phase III study and found the median survival for the CRS+HIPEC group was 11.0 months as compared to 6.5 months in the CRS alone group. Yonemura et al. (20) did a prospective randomized study on 139 patients with advanced gastric cancer who were treated with either chemohyperthermic peritoneal perfusion (CHPP) and surgery, chemonormothermic peritoneal perfusion (CNPP) and surgery, or surgery alone. They found that the 5-year OS rates for CHPP, CNPP and surgery alone groups were 61%, 43% and 42%, respectively. Fujimura et al. (21) did a randomized study that showed improved survival in patients receiving continuous hyperthermic peritoneal perfusion or continuous normothermic peritoneal perfusion as compared to the gastric surgery without perfusion group. Furthermore, the significant differences in the survival curves showed that peritoneal perfusion, whether hyperthermic or normothermic, is an effective procedure for preventing peritoneal recurrence. Yu et al. (22) did a prospective randomized trial which showed that gastric resection plus early postoperative intraperitoneal chemotherapy improved the 5-year OS compared with surgery only in patients with stage IV gastric cancer (28% and 5%, respectively).
There are many nonrandomized studies dealing with CRS and HIPEC. Fujimoto et al. (23) did a nonrandomized study and found increased survival rate for gastric cancer patients with peritoneal carcinomatosis receiving intraperitoneal hyperthermic chemoperfusion and aggressive surgery as compared to surgery alone. Hirose et al. (24) did a nonrandomized study to investigate the efficacy of continuous hyperthermic peritoneal perfusion with surgery as compared to surgery alone by a multivariate regression analysis. The continuous hyperthermic peritoneal perfusion group had higher median survival time and 1-year survival rate as compared to the control group: 11 months and 44.4% versus 6 months and 15.8%, respectively. Similarly, Yonemura et al. (25) found the median survival of 11.5 months in the patients who received cytoreduction and intraperitoneal hyperthermic chemotherapy (IPHC). Glehen et al. (26) found the median survival of patients receiving CRS followed by IPC to be 10.3 months with the median survival increasing to 21.3 months when a completeness of cytoreduction (CCR) score of CCR-0 or CCR-1 was obtained (Table 3).
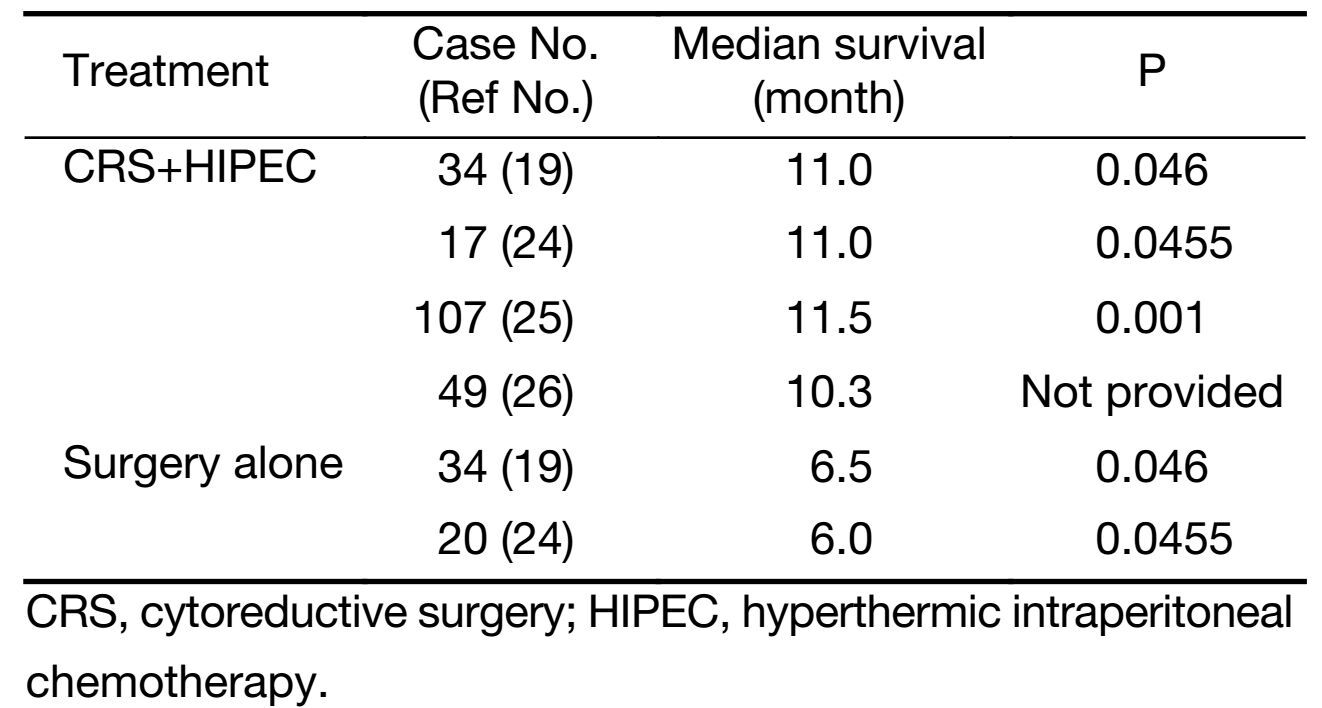
Full table
Complete versus incomplete cytoreduction
While CRS and HIPEC are promising treatment modalities for gastric cancer with peritoneal carcinomatosis, the success of the treatment is largely dependent on the resection status. Hall et al. (27) evaluated CRS and IPHC with peritoneal carcinomatosis from gastric cancer. The study investigated the outcomes of patients who underwent gastric resection with CRS followed by IPHC with mitomycin C, while the control group underwent radical gastrectomy without extended nodal resection. The OS was similar between the experimental and control groups (7.8 months and 8.0 months, respectively), and the OS time in the IPHC group was dependent on resection status. Within the IPHC group, a median survival time was 11.2 months if R0/R1 resection was completed as compared to 3.3 months if R2 resection was completed. Yonemura et al. (25) completed a retrospective study on 107 patients with peritoneal dissemination of gastric cancer who had intraoperative CHPP after CRS to see whether or not CCR or peritonectomy had an effect on OS and they found that the overall median survival was 11.5 months. The median survival was 15.5 months and 7.9 months in the complete and incomplete cytoreduction groups, respectively. The 5-year survival rate was 6.7% overall, and in the peritonectomy group, it was 27%. Scaringi et al. (28) found a 23.4 month median survival in patients without demonstrable peritoneal carcinomtosis who received CRS+HIPEC while the peritoneal carcinomatosis group had a median survival of 6.6 months. The median survival was 15 months when curative CRS was performed versus the median survival of 3.9 months in the palliative group. Similarly, Yang et al. (29) found that the median survival of patients with peritoneal cancer index (PCI) ≤20 undergoing CRS+HIPEC was 27.7 months while a PCI>20 had a median survival of 6.4 months. Thus, PCI>20 is an absolute contraindication for CRS-HIPEC in gastric cancer. Furthermore, PCI was found to predict the ability to achieve CCR-0, and thus survival. Yonemura et al. (30) determined that the best results are obtained with PCI<6. In another investigation, Chia et al. (31) also confirmed PCI<6 as a predictor of CCR-0. The estimated median survival for patients with CCR-0, CCR-1 and CCR-2/3 were 43.4 months, 9.5 months and 7.5 months, respectively (29). Glehen et al. (32) did a retrospective multicenter study to evaluate the treatment of CRS combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer. The study found an overall median survival of 9.2 months, while the median survival for completeness of CRS scores of CC-0, CC-1 and CC-2/3 was 15 months, 6 months and 4 months, respectively (Table 4).
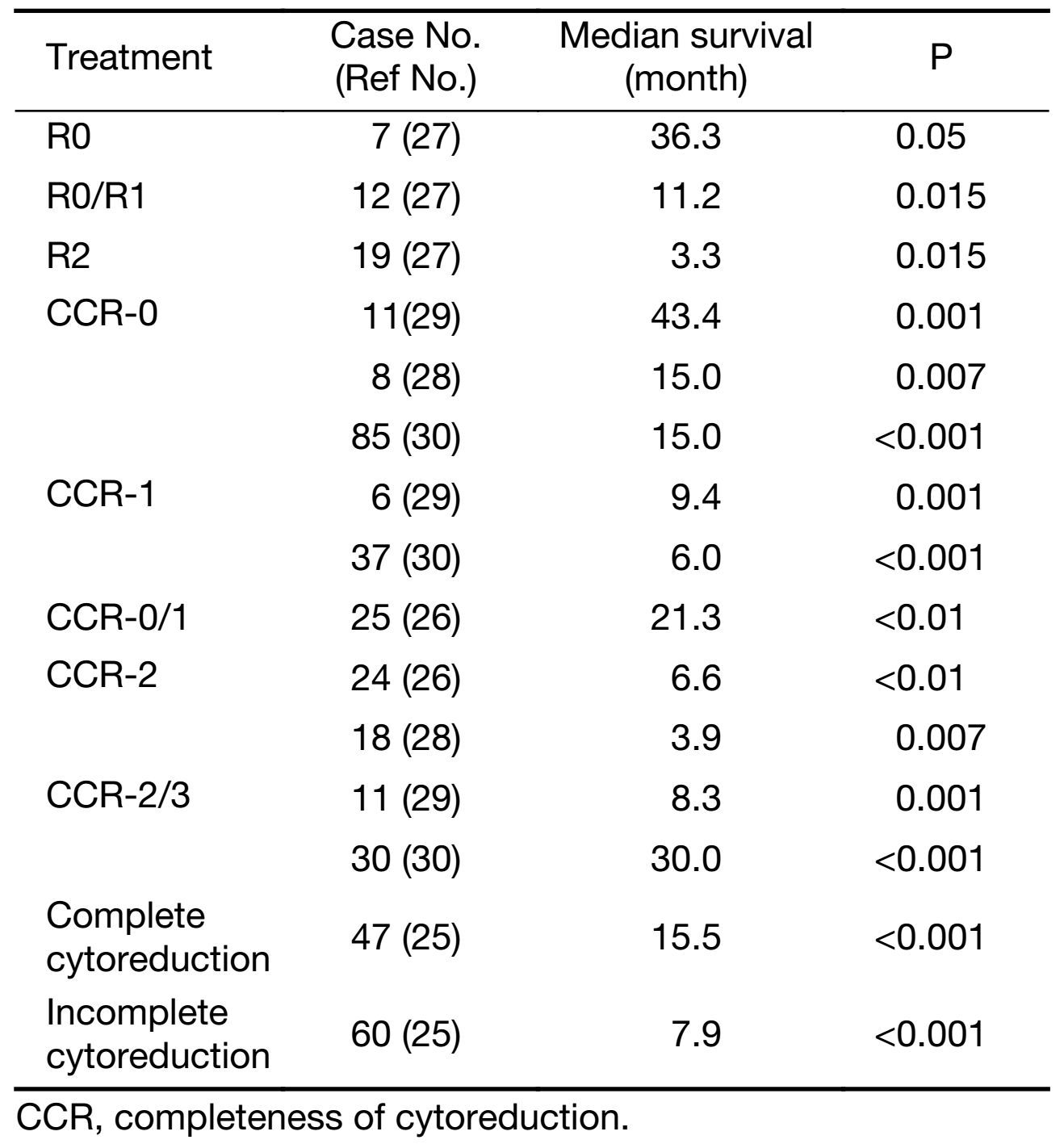
Full table
Quality of life (QOL)
Many practitioners argue that QOL after CRS-HIPEC is prohibitive. Tsilimparis et al. investigated QOL after CRS-HIPEC (33) and found that pre-and post-operative QOL did not differ statistically with most of the reduced elements recovering after 6−12 months. Zhu et al. (34) in a comprehensive review of QOL after CRS-HIPEC concluded that within 3 months 50% of patients returned to baseline QOL. We agree with other authors that reduced QOL of patients after CRS and HIPEC should not be used as an argument to deny surgical therapy to these patients.
Conclusions
Gastric cancer with peritoneal carcinomatosis has long been associated with a poor prognosis and is usually treated with palliative systemic chemotherapy. The state of the art treatment regimen including CRS and HIPEC has shown to increase OS rates. Gill et al. (35) did a systematic review of non-randomized, randomized, and prospective cohort trials regarding the effectiveness of CRS and HIPEC in patients with gastric cancer and peritoneal carcinomatosis and found that the overall median survival was 7.9 (range: 6.1−9.2) months and improved to 15 months if the patients had a CCR score of 0 or 1. They also found that the 1-year survival rate was 43% (range: 22%−8%). Although there is a higher perioperative morbidity and mortality associated with CRS and HIPEC, patients should not be dissuaded because it has been found that the postoperative QOL was similar to that of preoperative by 6−12 months (36). Surgeons' proficiency has a major impact on outcomes in CRS-HIPEC. Rahul et al. (37) reviewed extensively the subject and concluded that the learning curve for a center is approximately 140−220 cases, and for individual surgeons about 33−70 cases. Our recommendations are: CRS-HIPEC for gastric cancer should be contemplated in a multidisciplinary manner for physically fit patients [Eastern Cooperative Oncology Group (ECOG) performance status score of 0/1 without significant comorbidities] who underwent staging laparoscopy with PCI<6 and to be performed by experienced surgeons.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon: IARC, 2013. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133–45. [PubMed] DOI:10.1002/ijc.v132.5
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [PubMed] DOI:10.3322/caac.v61:2
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual.7th edition.New York: Springer, 2010.
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–363. [PubMed] DOI:10.1002/(ISSN)1097-0142
- Fanelli MF, de Paiva TF Jr, Silva MJ, et al. Predictors of peritoneal carcinomatosis in patients with gastric cancer treated at a single institution in Brazil. J Surg Oncol 2009;100:452–5. [PubMed] DOI:10.1002/jso.v100:6
- Tanaka T, Kumagai K, Shimizu K, et al. Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J Surg Oncol 2000;75:165–71. [PubMed] DOI:10.1002/(ISSN)1096-9098
- Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003;90:1113–9. [PubMed] DOI:10.1002/(ISSN)1365-2168
- Kim KW, Chow O, Parikh K, et al. Peritoneal carcinomatosis in patients with gastric cancer, and the role for surgical resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy. Am J Surg 2014;207:78–83. [PubMed] DOI:10.1016/j.amjsurg.2013.04.010
- Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622–8. [PubMed] DOI:10.1002/ijc.28373
- Bozzetti F, Yu W, Baratti D, et al. Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol 2008;98:273–6. [PubMed] DOI:10.1002/jso.v98:4
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;17:Cd004064. [PubMed] DOI:10.1002/14651858.CD004064.pub3
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Oba K, Paoletti X, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer 2013;49:1565–77. [PubMed] DOI:10.1016/j.ejca.2012.12.016
- Hioki M, Gotohda N, Konishi M, et al. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg 2010;34:555–62. [PubMed] DOI:10.1007/s00268-010-0396-5
- Kerkar SP, Kemp CD, Duffy A, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials 2009;10:121. [PubMed] DOI:10.1186/1745-6215-10-121
- Hultman B, Lundkvist J, Glimelius B, et al. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol 2012;51:112–21. [PubMed] DOI:10.3109/0284186X.2011.594809
- Ikeguchi M, Oka A, Tsujitani S, et al. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res 1994;14:2131–4. [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242–6. [PubMed] DOI:10.1097/SLA.0b013e3181b0c80e
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81. [PubMed] DOI:10.1245/s10434-011-1631-5
- Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastro-enterology 2001;48:1776–82. [PubMed]
- Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 1994;18:150–5. [PubMed] DOI:10.1007/BF00348209
- Yu W, Whang I, Chung HY, et al. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 2001;25:985–90. [PubMed] DOI:10.1007/s00268-001-0067-7
- Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997;79:884–91. [PubMed] DOI:10.1002/(ISSN)1097-0142
- Hirose K, Katayama K, Iida A, et al. Efficacy of continuous hyperthermic peritoneal perfusion for the prophylaxis and treatment of peritoneal metastasis of advanced gastric cancer: evaluation by multivariate regression analysis. Oncology 1999;57:106–14. [PubMed] DOI:10.1159/000012016
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370–5. [PubMed] DOI:10.1002/(ISSN)1365-2168
- Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004;139:20–6. [PubMed] DOI:10.1001/archsurg.139.1.20
- Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454–63. [PubMed] DOI:10.1016/j.gassur.2003.12.014
- Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 2008;34:1246–52. [PubMed] DOI:10.1016/j.ejso.2007.12.003
- Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol 2010;101:457–64. [PubMed] DOI:10.1002/jso.v101:6
- Yonemura Y, Ishibashi H, Hirano M, et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol 2017;24:478–85. [PubMed] DOI:10.1245/s10434-016-5487-6
- Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 2016;23:1971–9. [PubMed] DOI:10.1245/s10434-015-5081-3
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370–7. [PubMed] DOI:10.1245/s10434-010-1039-7
- Tsilimparis N, Bockelmann C, Raue W, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol 2013;20:226–32. [PubMed] DOI:10.1245/s10434-012-2579-9
- Zhu Y, Hanna N, Boutros C, et al. Assessment of clinical benefit and quality of life in patients undergoing cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for management of peritoneal metastases. J Gastrointest Oncol 2013;4:62–71. [PubMed] DOI:10.3978/j.issn.2078-6891.2012.053
- Gill RS, Al-Adra DP, Nagendran J, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol 2011;104:692–8. [PubMed] DOI:10.1002/jso.v104.6
- Tsilimparis N, Bockelmann C, Raue W, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol 2013;20:226–32. [PubMed] DOI:10.1245/s10434-012-2579-9
- Rajeev R, Klooster B, Turaga KK, et al. Impact of surgical volume of centers on post-operative outcomes from cytoreductive surgery and hyperthermic intra-peritoneal chemoperfusion. J Gastrointest Oncol 2016;7:122–8. [PubMed] DOI:10.3978/j.issn.2078-6891.2015.099
