Helicobacter pylori antibody responses in association with eradication outcome and recurrence: a population-based intervention trial with 7.3-year follow-up in China
Introduction
Helicobacter pylori (H. pylori) infection has been recognized as the leading cause of chronic gastritis, peptic ulcer disease and gastric cancer (1,2). Accumulating evidences indicated that anti-H. pylori treatment could reduce the risks of precancerous gastric lesions and gastric cancer (3-6). Therefore, gastric cancer prevention by eradication of H. pylori in a high risk population is highly encouraged (7).
Although new regimens have been developed to eradicate H. pylori infection, two challenges existed, failure of eradication and recurrence of the infection (8,9). Currently, even under conventional H. pylori eradication therapy, there were still 10%–30% failure (8,10). Moreover, the annual recurrence rates after eradication were about 3.4% in developed countries and 8.7% in developing countries, respectively (9). The short- and long-term impacts on anti-H. pylori treatment may require a relevant monitor to evaluate biological effectiveness of the eradication and recurrence, particularly in a large community-based gastric cancer intervention.
13C-urea breath test (13C-UBT) is currently a golden standard to identify H. pylori infection but it requires specific devices and the cost is relatively high (11). And alternative to 13C-UBT, serum biomarker is an area of investigation, particularly for H. pylori specific antibodies. Although the sensitivity and specificity to evaluate the status of H. pylori infection by antibody responses are relatively lower than 13C-UBT, it is more feasible, practical, and cost-effective in a large scale population-based anti-H. pylori treatment (12). In addition, it is also interesting to know whether certain serum H. pylori antibodies are more specifically associated with the outcomes of H. pylori treatment, which could be potential markers for targeting population at a high risk of gastric cancer.
Our previous Shandong Intervention Trial in Linqu County (SIT, clinicaltrials.gov identifier: NCT00339768), one of the highest risk areas of gastric cancer in the world, provided us a unique opportunity to conduct such investigation (3,13,14). This 7.3-year follow-up trial allowed us to dynamically assess the total anti-H. pylori immunoglobulin G (IgG) and specific antibody levels before and after anti-microbial treatment, which may serve as a surrogate monitor of 13C-UBT to evaluate long-term risk of recurrence, and short-term impact on the effectiveness of eradication.
Materials and methods
Study subjects
Details of this trial were described in elsewhere (3,15). Briefly, 3,365 eligible trial participants aged 35–64 years were recruited from 13 villages selected at random to conduct a randomized, double-blind, placebo-controlled intervention trial in 1995. The H. pylori infection status of each individual was determined by serum H. pylori antibody test at baseline. Amoxicillin and omeprazole treatment of H. pylori infection or the corresponding placebos were randomly assigned to 2,258 H. pylori seropositive subjects in 1995. Among them, a total of 1,803 trial participants received a 13C-UBT at 45-d after the completion of antibiotic treatment and at the end of this trial in 2003. In the meantime, total serum anti-H. pylori antibody levels were parallelly determined among the same trial participants at 1-year (in 1996), 2-year (in 1997) and 7.3-year (in 2003) after anti-H. pylori treatment, respectively.
For the current study, we further randomly selected 473 participants from active treatment group (908 eligible subjects) and 56 participants from placebo group (895 eligible subjects), to evaluate serum total anti-H. pylori IgG and 10 specific antibody levels before and after treatment at 1-, 2- and 7.3-year. This study was approved by the Institutional Review Board of Peking University Cancer Hospital, and written informed consent was provided by all participants in the study.
Questionnaires
At study enrollment, all participants were interviewed in person by trained interviewers using a structured questionnaire, consisting of the following sections: 1) demographic background; 2) personal habits; 3) medical history; and 4) dietary habits (3,15).
13C-UBT
Details of 13C-UBT were described previously (15). In brief, participants fasted overnight before baseline exhaled CO2 samples were collected. Expired gas was collected 30 min later after drinking 20 mL water with a pill of 80 mg 13C-urea (>99%) (Baylor Medical College, Houston, Texas, USA).13CO2 values were determined by a gas isotopic ratio mass spectrometry (Finnigan MAT, Bremen, Germany), and any concentration of 13CO2 at 30 min that exceeded the baseline concentration by more than 0.6% was regarded as a positive result (15). The sensitivity and specificity of this test were 93.1% and 95.7%, respectively (16).
Enzyme-linked immunosorbent assay (ELISA)
Serum total H. pylori IgG antibodies were evaluated by ELISA as previously described (17). Cultured from two patients’ gastric biopsies in Linqu County, H. pylori strains were used as a group antigen to provide the antigenic preparation for serology (17). The protein concentrations were measured by the modification of the Lowry method, and the soluble material from the two strains was pooled for the ELISA procedure (18,19). All assays were done in duplicate on coded samples and the optical density was measured with a commercial ELISA reader (Bio-Rad, Hercules, California, USA). The mean absorbance (optical density) for IgG was used for quantification of antibody titers. An individual was determined to be positive for H. pylori infection if the mean optical density for IgG exceeded 1.0, a cut-off point based on the H. pylori negative subjects and reference sera (17). Appropriate blanks, positive and negative controls were included in each assay as described (19-21). The intra-assay and inter-assay variation were less than 10%, as estimated with positive and negative control sera. Quality-control samples were assayed at Vanderbilt University, Nashville, Tennessee, USA. This method yielded an estimated sensitivity of 100% and specificity of 94.9% (15,19,22-24).
RecomLine H. pylori IgG
The recomLine H. pylori IgG is a line immune assay (Mikrogen, Munich, Germany) based on recombinantly expressed H. pylori proteins that, in contrast to ELISA, allows the identification of specific antibody responses against distinct H. pylori antigens (25). All H. pylori recombinant proteins were derived from strain J99. This method was applied to simultaneously detect 10 highly immunogenic recombinant H. pylori antigens (CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA, HP0231, and CtkA). Compared with histology findings, this assay yielded an estimated sensitivity of 99.3% and specificity of 100% (26).
Statistical analysis
Differences of baseline characteristics across various outcome groups after H. pylori eradication were compared using Chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables.
To assess different serum H. pylori antibody levels associated with eradication outcome and recurrence, we further classified the 473 trial participants in active arm into three groups according to the results of 13C-UBT: failed eradication (13C-UBT positive at 45-d after treatment; n=140); recurrence (13C-UBT negative at 45-d and positive at 7.3-year after treatment; n=156); and sustainably eradicated groups (13C-UBT negative at 45-d and 7.3-year after treatment; n=177). Because the distributions of serum H. pylori antibody levels were skewed, we used medians of the antibody to present the levels of each group.
Associations between serum H. pylori specific seropositive antibodies and H. pylori eradication outcomes were estimated by odds ratios (ORs) and 95% confidence interval (95% CI) using unconditional multiple logistic regression adjusting age and gender. The Bonferroni correction was applied to recognize P values at <0.005 (0.05/10 markers). Education, smoking, and drinking were evaluated as the potential confounders but not included in the final models, because they did not substantially alter the risk estimates (data not shown). We calculated Spearman’s correlation coefficients (r) to assess colinearity between different H. pylori specific antibodies. Tests for linear trend were performed by entering the categorical variables as continuous parameters in the models. Effect modification by specific antibodies was calculated using a likelihood ratio test to compare models with and without interaction terms. All statistical analyses were conducted with SAS software (Version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
A total of 529 trial participants were included in the present study with a median age of 43.0 years old at baseline. The dominant trial participants were female (57.8%), non-smokers (64.5%), non-drinkers (57.8%), and had low education background (80.2% with elementary school education or less). Among the three active anti-H. pylori treatment groups and placebo group, participants in failed eradication group were younger (P=0.004) and male (P=0.016) (Table 1). No statistically significant differences were observed for education, smoking and drinking between groups (P>0.05).

Full table
The dynamic changes of total anti-H. pylori IgG titers were compared among different groups during the 7.3-year follow-up (Figure 1). The medians of IgG titers at baseline in 1995 showed no significant difference among four groups (3.2 in placebo, 2.8 in failed eradication, 2.9 in sustainably eradicated, and 3.0 in recurrence group, P=0.142) (Supplementary Table 1). However, after treatment, the medians of IgG titers remained at the highest levels in the placebo group throughout the follow-up period (3.1 at 1-year, 4.2 at 2-year and 4.9 at 7.3-year after treatment, respectively). Similar trend was also observed in failed eradication group (2.5 at 1-year, 3.3 at 2-year and 3.9 at 7.3-year). For subjects in sustainably eradicated group, the medians of IgG titers declined rapidly from 2.9 at baseline to 0.7 at 1-year after successful eradication, remaining at 0.6 both at 2- and 7.3-year after treatment. On the contrary, the medians of IgG titers in recurrence group dropped to 1.2 (slightly higher than the cutoff value of 1.0) at 1-year, 1.3 at 2-year and 2.0 at 7.3-year after treatment, respectively.
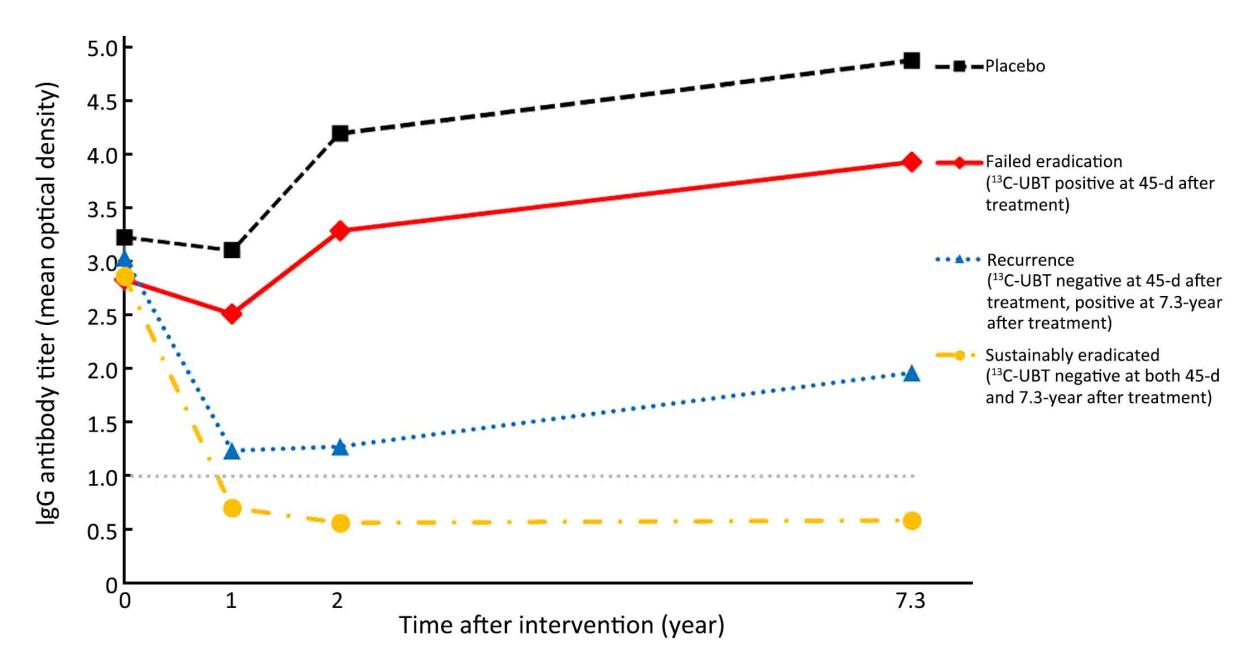
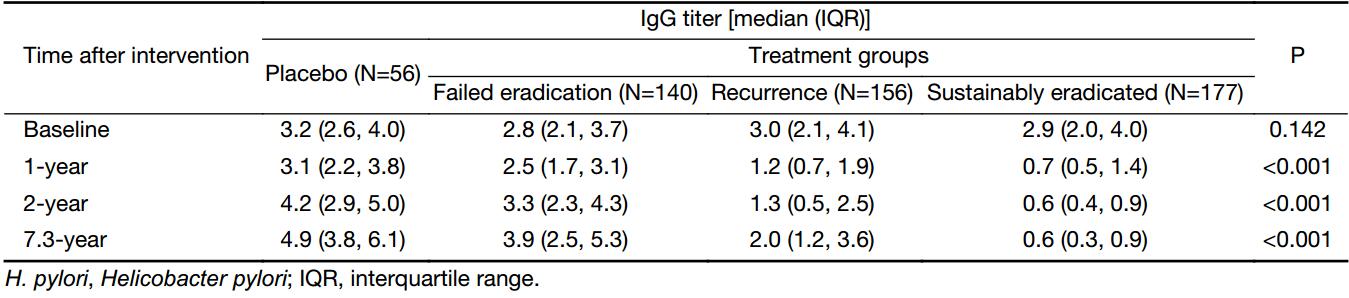
Full table
We further examined the distribution of specific antibodies against distinct H. pylori antigens in each group (Table 2). Compared with sustainably eradicated group, the frequencies of FliD and HpaA seropositivities of the 10 individual H. pylori proteins included in the biomarker panel at baseline were significantly lower in failed eradication group [for FliD, OR=0.44; 95% CI, 0.27–0.73; for HpaA, OR=0.32; 95% CI, 0.17–0.60; adjusting for multiple comparisons with α at 0.005 (0.05/10 markers)]. When grouping individuals into four categories by these two factors, participants co-positive to two proteins showed further less risk of failed eradication compared to co-negative subjects (OR=0.27; 95% CI, 0.14–0.54). While no interaction was found between FliD and HpaA on the risk of failed eradication (Pinteraction=0.843).
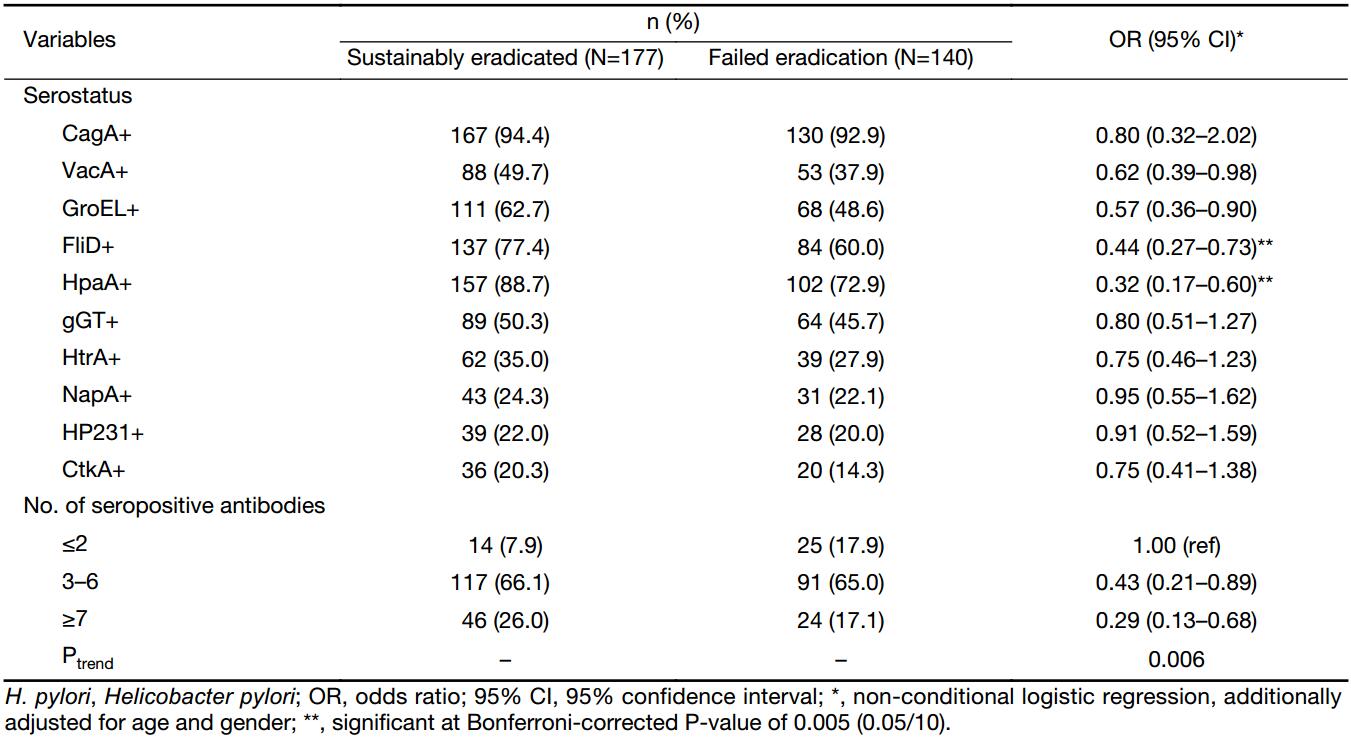
Full table
We also compared the number of positive antibodies in this panel at baseline between sustainably eradicated and failed eradication groups (Table 2). Individuals with combination of three to six seropositive antibodies showed a declined risk of failed eradication than those with up to two antibodies (OR=0.43; 95% CI, 0.21–0.89), and the risk further decreased for those with more than six positive antibodies (OR=0.29; 95% CI, 0.13–0.68). Increasing number of seropositive antibodies was associated with a decreased OR for failed eradication in a linear fashion (Ptrend=0.006).
Nevertheless, with the same analysis strategy, no association was found between baseline seropositivities and H. pylori recurrence (Table 3). Although we did observe a decreased risk of recurrence for those with three to six seropositive antibodies (OR=0.45; 95% CI, 0.22–0.92), the association was attenuated among individuals with more than six antibodies (OR=0.60; 95% CI, 0.28–1.32).
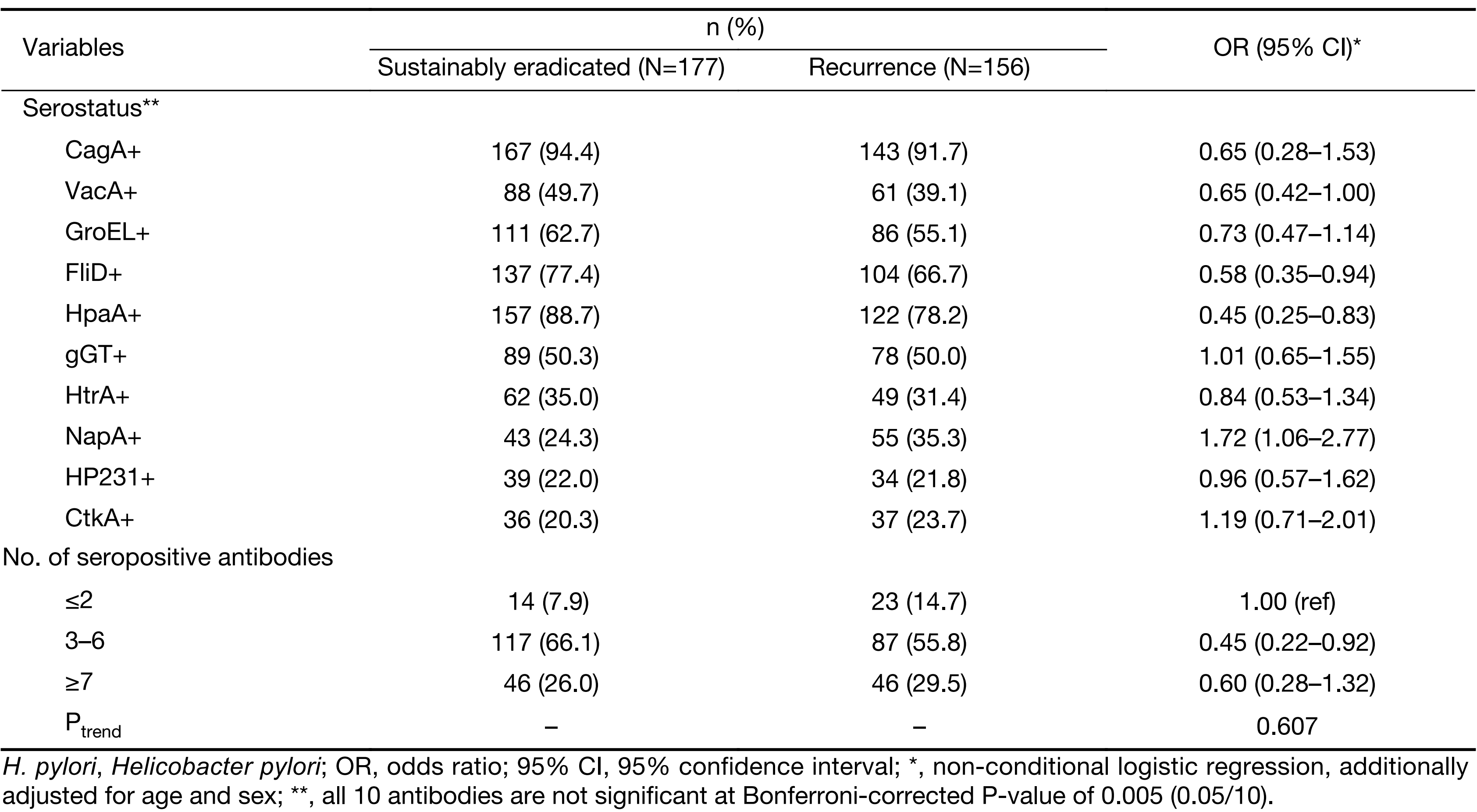
Full table
The dynamic changes of specific antibody levels were further explored between sustainably eradicated and recurrence groups. In sustainably eradicated group, the percentages of seropositivity for all 10 antibodies decreased significantly in the first year after treatment (all P<0.001), with the relative differences ranging from 22.6% for CagA to 63.0% for HtrA (Figure 2). In the 7.3-year after treatment, the decreases of all antibodies were further enlarged, from 48.2% for HpaA to 81.0% for FliD (all P<0.001). For the recurrence group, no significant decrease was found for the frequencies of CagA and VacA seropositivity in the first year after eradication (P=0.346 and 0.061 for CagA and VacA, respectively), which was followed by delayed decreases in the 7.3 years (P<0.001 and P=0.033 for CagA and VacA, respectively). The percentages of the other eight specific H. pylori antibodies all decreased significantly at 1-year after treatment, while no additional decline was observed in the 7.3-year. Seropositivies of HpaA and HP231 even rebounded and showed no difference with baseline (for HpaA, P=0.289; for HP231, P=0.201). The comparison of the seropositivity alteration between these two outcome groups found fewer participants reversed from positive to negative in recurrence group. Statistical significances were observed for six antibodies (CagA, VacA, GroEL, FliD, HpaA, and HtrA) in the 1-year after treatment (all P<0.05), and additional three (NapA, HP231, and CtkA) in the 7.3-year.
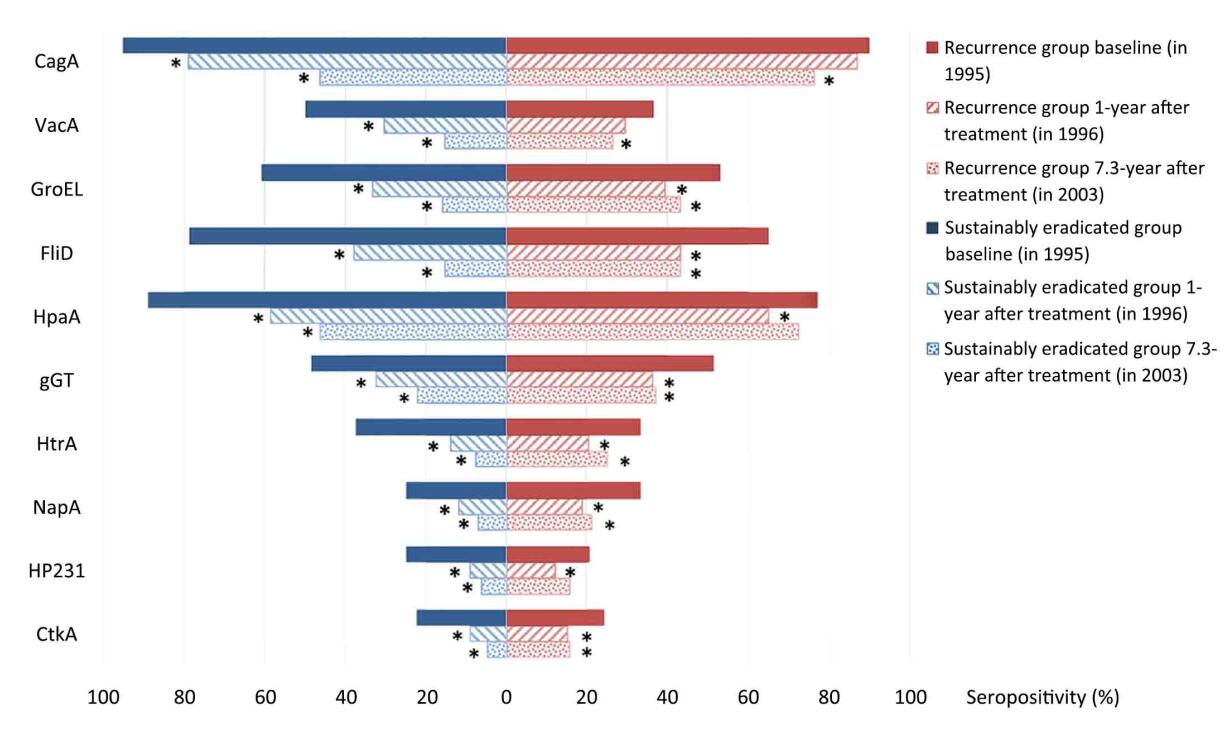
Discussion
In our population-based study, utilizing the serum samples collected at different time points during the follow-up in an intervention trial, the total anti-H. pylori IgG and 10 specific antibody levels were determined to evaluate the association between H. pylori antibody responses and outcomes of anti-H. pylori treatment. We found that the medians of anti-H. pylori IgG titers were consistently below cut-off value of 1.0 throughout the 7.3-year follow-up period in eradicated group. While relative elevated H. pylori IgG antibody levels at 1-year after treatment may indicate increasing risk of recurrence. Moreover, our study also suggested that multiple seropositivities of specific H. pylori antibodies at baseline, especially FliD and HpaA, may serve as serum biomarkers to predict a better outcome of eradication.
By dynamically investigating total anti-H. pylori IgG titers at several time points before and after interventions, our present study found significantly different alteration tendency in various outcome groups. Compared to the persistent high levels of serum IgG in placebo and failed eradication groups as expected, significant declines were noticed at 1-year after eradication in recurrence and sustainably eradicated groups. Although previous studies have evaluated serum IgG levels as the recurrence monitor, our study added the new evidence in a long-term follow up for its predictive significance in a population-based prevention (27,28). In our study, the relative higher median of IgG titers at 1-year after eradication in recurrence group may provide a warning of recurrence risk. Interestingly, medians of IgG titers from 1-year to 7.3-year after treatment always kept at a lower level in recurrence group compared to those in failed eradication groups. One possibility was that new infection might not occur thereafter, instead, relapse of silent H. pylori after anti-bacterial treatment could lead to the recurrence (9). The low serum antibody responses shown by this serological result may suggest lesser extent and density of H. pylori excising in gastric mucosa, while majority of H. pylori bacteria was eliminated (29). These important findings may explain our previous finding in this trial that, even subjects with recurrence of H. pylori, long-term beneficial effects could also be obtained for reduction of gastric cancer risk (30).
Besides the total IgG, the specific antibodies against distinct H. pylori proteins at baseline were also investigated for the predictive significances of short and long term eradication outcome. A recent meta-analysis suggested the absence of CagA, one of the most important bacterial virulence factors for peptic ulcer disease and gastric carcinoma, may serve as a predictor for failure of H. pylori eradication [risk ratio (RR)=2.0; 95% CI, 1.6–2.4], which was not confirmed in our study (OR=1.25; 95% CI, 0.50–3.16) (31). While in this meta-analysis, none of the 14 selected studies were conducted in East Asia. Accumulating studies in East Asia, including ours, reported more than 90% of infected H. pylori strains were CagA positive, which may not be used as a helpful marker in this population (32-34).
Instead of CagA, absence of FliD or HpaA antibodies at baseline was found as independent predictors for failure of H. pylori eradication in the Chinese population. The H. pylori flagella hook-associated protein 2 homologue, FliD, is essential in the assembly of the functional flagella, which not only plays a vital role in bacterial motility, but also is necessary for colonization and persistence of H. pylori infection (35,36). Known as H. pylori adhesin A, HpaA is a surface-located lipoprotein, which is essential for the colonization of H. pylori (37-40). Recognized by human dendritic cells, HpaA could induce their maturation and antigen presentation (41). Although the mechanisms of FliD and HpaA with the tendency of successful eradication are still unknown, it may involve dysfunction of colonization by reducing the adhesion of H. pylori to gastric mucosa in the absence of these two proteins.
Not only the specific antibodies like FliD and HapA, increasing number of various seropositive H. pylori antibodies at baseline was also found to be associated with successful eradication outcome in a linear fashion. According to our previous results from the same intervention trial, the subjects with increasing number of H. pylori seropositive antibodies at baseline showed a higher risk for progression to gastric cancer (42). Both of these prospective studies suggested a potential preferential population for anti-H. pylori treatment in the subjects with multiple seropositive antibodies, especially FliD and HapA, for their higher risk of gastric cancer and easier eradication tendency.
Although no significant association was found between baseline seropositivities and H. pylori recurrence risk, the dynamic changes of specific antibodies levels showed fewer participants reversed from positive to negative in recurrence group. For the subjects recurring H. pylori infection in the long follow-up period, seropositivities of CagA and VacA still remained at high levels at 1-year after successful eradication and showed delayed declines in 7.3-year. The discrepant change trends of CagA or VacA seropositivity in recurrence group from sustainably eradicated subjects may also suggest potential indicators of recurrence risk.
Certain limitations of the present study should be considered in interpreting the results. In recent studies, H. pylori related serum biomarkers such as Omp, HP 0305, HyuA, and HcpC were found to be associated with gastric cancer risk (34,43,44). However, our biomarker panel included only 10 H. pylori-specific antibodies, more defined markers need to be further investigated. Nevertheless, we employed CagA and VacA as the two most important virulence factors, and GroEL, HtrA, NapA, HP231, CtkA for their close associations with severe gastric diseases (45-48). Moreover, blood samples were only performed at baseline and 1, 2, 7.3 years after intervention in our follow-up study. The detailed fluctuation of antibodies levels could not be observed between two and seven years after treatment, and the exact recurrence time point could not be identified in the current cohort. To better elucidate these problems, further monitor with more frequent follow-up time points is needed in the future.
Our study has a number of strengths. Most notably, to our knowledge, it is the first study to assess multiple serology to H. pylori individual antibodies at several time points both before and after treatment, and its association with short- and long-term anti-H. pylori treatment outcome in the high-risk population of gastric cancer in China. We took advantage of stored serum samples and rich covariate information from a strictly conducted population-based intervention trial in China. Furthermore, the results from 13C-UBT, recomLine H. pylori IgG, and conventional ELISA in the same population enabled us to explore their differing applications at each time point. Additionally, the long-term follow-up in our study enabled the accurate evaluation of eventual eradication outcome.
Conclusions
Our long-term follow-up intervention trial based study in the high-risk area of China confirmed the previous research and provided additional evidence that H. pylori antibodies could be used as a potential indicator for H. pylori recurrence risk. Subjects with multiple seropositive specific antibodies at baseline, especially FliD and HpaA, may be associated with better anti-H. pylori treatment outcome. The results of this study suggested potential targeted strategies for anti-H. pylori treatment in a gastric cancer high-risk population in China with different distribution of H. pylori strains from Western countries.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (No. 81171989, 30801346), National Basic Research Program of China (973 Program: 2010CB529303), and the Capital Health Research and Development of Special (2014-2-1022).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer . Int J Cancer 2004;109:138–43. [PubMed] DOI:10.1002/ijc.11680
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts . Gut 2001;49:347–53. [PubMed]
- You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J NatI Cancer Inst 2006;98:974–83. [PubMed] DOI:10.1093/jnci/djj264
- Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial . JAMA 2004;291:187–94. [PubMed] DOI:10.1001/jama.291.2.187
- Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial . Lancet 2008;372:392–7. [PubMed] DOI:10.1016/S0140-6736(08)61159-9
- Ley C, Mohar A, Guarner J, et al. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double-blind, placebo-controlled trial . Cancer Epidemiol Biomarkers Prev 2004;13:4–10. [PubMed]
- IARC Helicobacter pylori Working Group (2014).Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). Available online: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php
- van der Hulst RW, Keller JJ, Rauws EA, et al. Treatment of Helicobacter pylori infection: a review of the world literature . Helicobacter 1996;1:6–19. [PubMed]
- Gisbert JP. The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review . Am J Gastroenterol 2005;100:2083–99. [PubMed] DOI:10.1111/j.1572-0241.2005.50043.x
- Pan KF, Zhang L, Gerhard M, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication . Gut 2016;65:9–18. [PubMed] DOI:10.1136/gutjnl-2015-309197
- Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection — a critical review . Aliment Pharmacol Ther 2004;20:1001–17. [PubMed] DOI:10.1111/j.1365-2036.2004.02203.x
- Ho B, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Serologic testing . Gastroenterol Clin North Am 2000;29:853–62. [PubMed]
- Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality . J NatI Cancer Inst 2012;104:488–92. [PubMed] DOI:10.1093/jnci/djs003
- You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res 1993;53:1317–21. [PubMed]
- Gail MH, You WC, Chang YS, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Control Clin Trials 1998;19:352–69. [PubMed]
- Jiang J, Wang SZ, Li XM, et al. The modification of 13C-urea breath test. Zhonghua He Yi Xue Zai Zhi (in Chinese) 1994;14:103–5.
- Zhang L, Blot WJ, You WC, et al. Helicobacter pylori antibodies in relation to precancerous gastric lesions in a high-risk Chinese population . Cancer Epidemiol Biomarkers Prev 1996;5:627–30. [PubMed]
- Markwell MA, Haas SM, Bieber L, et al. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 1978;87:206–10. [PubMed]
- Perez-Perez GI, Dworkin BM, Chodos JE, et al. Campylobacter pylori antibodies in humans . Ann Intern Med 1988;109:11–7. [PubMed]
- Perez-Perez GI, Marrie T, Inouye H, et al. The effect of age and occupation on the seroprevalence of Helicobacter pylori infection . Can J Infect Dis 1992;3:134–8. [PubMed] DOI:10.1155/1992/831523
- Pérez-Pérez GI, Thiberge JM, Labigne A, et al. Relationship of immune response to heat-shock protein A and characteristics of Helicobacter pylori — infected patients . J Infect Dis 1996;174:1046–50. [PubMed]
- Drumm B, Perez-Perez GI, Blaser MJ, et al. Intrafamilial clustering of Helicobacter pylori infection . N Engl J Med 1990;322:359–63. [PubMed] DOI:10.1056/NEJM199002083220603
- Glassman MS, Dallal S, Berezin SH, et al. Helicobacter pylori-related gastroduodenal disease in children. Diagnostic utility of enzyme-linked immunosorbent assay . Dig Dis Sci 1990;35:993–7. [PubMed]
- Groves FD, Zhang L, Li JY, et al. Comparison of two enzyme-linked immunosorbent assay tests for diagnosis of Helicobacter pylori infection in China . Cancer Epidemiol Biomarkers Prev 1997;6:551–2. [PubMed]
- Formichella L, Romberg L, Bolz C, et al. A novel line immunoassay based on recombinant virulence factors enables highly specific and sensitive serologic diagnosis of Helicobacter pylori infection . Clin Vaccine Immunol 2013;20:1703–10. [PubMed] DOI:10.1128/CVI.00433-13
- Nölting C, Reichhuber C, Formichella L, et al. Development and validation of a novel line immunoassay for diagnosis of Helicobacter pylori infection. Z Gastroenterol 2016;54-KV417.
- Lee JH, Kim N, Chung JI, et al. Long-term Follow up of Helicobacter pylori IgG serology after eradication and reinfection rate of H. pylori in South Korea . Helicobacter 2008;13:288–94. [PubMed] DOI:10.1111/j.1523-5378.2008.00616.x
- Wang WM, Chen CY, Jan CM, et al. Long-term follow-up and serological study after triple therapy of Helicobacter pylori-associated duodenal ulcer . Am J Gastroenterol 1994;89:1793–6. [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [PubMed]
- Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J NatI Cancer Inst 2014;106. Pii:dju116.
- Suzuki T, Matsuo K, Sawaki A, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection . Aliment Pharmacol Ther 2006;24:273–80. [PubMed] DOI:10.1111/j.1365-2036.2006.02994.x
- Annibale B, Lahner E, Santucci A, et al. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis . Helicobacter 2007;12:23–30. [PubMed] DOI:10.1111/j.1523-5378.2007.00467.x
- Fusconi M, Vaira D, Menegatti M, et al. Anti-CagA Reactivity in Helicobacter pylori-negative subjects: a comparison of three different methods . Dig Dis Sci 1999;44:1691–5. [PubMed]
- Cai H, Ye F, Michel A, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia . Int J Epidemiol 2016;45:774–81. [PubMed] DOI:10.1093/ije/dyw078
- Eaton KA, Suerbaum S, Josenhans C, et al. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes . Infect Immun 1996;64:2445–8. [PubMed]
- Khalifeh Gholi M, Kalali B, Formichella L, et al. Helicobacter pylori FliD protein is a highly sensitive and specific marker for serologic diagnosis of H. pylori infection . Int J Med Microbiol 2013;303:618–23. [PubMed] DOI:10.1016/j.ijmm.2013.08.005
- Carlsohn E, Nyström J, Bölin I, et al. HpaA is essential for Helicobacter pylori colonization in mice . Infect Immun 2006;74:920–6. [PubMed] DOI:10.1128/IAI.74.2.920-926.2006
- Blom K, Lundin BS, Bölin I, et al. Flow cytometric analysis of the localization of Helicobacter pylori antigens during different growth phases . FEMS Immunol Med Microbiol 2001;30:173–9. [PubMed]
- Lundström AM, Blom K, Sundaeus V, et al. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains . Microb Pathog 2001;31:243–53. [PubMed] DOI:10.1006/mpat.2001.0466
- O’Toole PW, Janzon L, Doig P, et al. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein . J Bacteriol 1995;177:6049–57. [PubMed]
- Voland P, Hafsi N, Zeitner M, et al. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori . Infect Immun 2003;71:3837–43. [PubMed]
- Pan KF, Formichella L, Zhang L, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population . Int J Cancer 2014;134:2118–25. [PubMed] DOI:10.1002/ijc.28560
- Gao L, Michel A, Weck MN, et al. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H. pylori proteins determined by novel multiplex serology . Cancer Res 2009;69:6164–70. [PubMed] DOI:10.1158/0008-5472.CAN-09-0596
- Epplein M, Zheng W, Xiang YB, et al. Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men . Cancer Epidemiol Biomarkers Prev 2012;21:2185–92. [PubMed] DOI:10.1158/1055-9965.EPI-12-0792-T
- Formichella L, Göttner G, Nölting C, et al. Correlation of IgG immune responses to selected H. pylori proteins with disease status in different populations. Poster presented at the XXVIIth International Workshop of the European Helico-bacter Study Group (2014).
- Formichella L, Romberg L, Vieht M, et al. Evaluation of IgG immune responses to 15 H. pylori proteins. Poster presented at the XXVIth International Workshop of the European Helicobacter Study Group (2013).
- Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach . Cancer Res 1995;55:2111–5. [PubMed]
- Basso D, Navaglia F, Brigato L, et al. Analysis of Helicobacter pylori vacA and cagA genotypes and serum antibody profile in benign and malignant gastroduodenal diseases . Gut 1998;43:182–6. [PubMed]
