Secretory carcinoma — impact of translocation and gene fusions on salivary gland tumor
Introduction
Secretory carcinoma (SC), previously known as mammary analogue secretory carcinoma (MASC), is a new entity of malignant salivary gland tumor, which is similar to mammary SC in terms of its histologic and genetic features (1,2). Mammary SC is a rare malignancy and is reported to show slow-growing and indolent behavior. It occurs more often in younger women than in elderly women (3). Molecularly, mammary SC is the only epithelial tumor with the t(12;15)(p13;q25) translocation in breast, leading to a fusion of the ETV6 gene from chromosome 12 and the NTRK3 gene from chromosome 15 (4,5). The ETV6-NTRK3 fusion gene encodes a chimeric tyrosine kinase, and this fusion results in a constitutively active chimeric tyrosine kinase mitogenic pathway and the phosphatidyl inositol-3-kinase (PI3K)-AKT pathway (6,7). In 2010, Skálová et al. reviewed 16 salivary gland tumor cases that were previously classified as either acinic cell carcinoma (AciCC) or adenocarcinoma not otherwise specified (ADC-NOS) (8). The authors found that all cases but one were also positive for the ETV6-NTRK3 translocation. They specified these cases as MASC, indicating a new salivary gland tumor entity. Since the first description of SC/MASC, more than 100 additional cases have been reported (8-20).
Clinical features
Many cases of SC/MASC had been previously classified as AciCC, mucoepidermoid carcinoma (MEC), and ADC-NOS. The most common malignancies reclassified as SC were ADC-NOS (37.8%) and AciCC (12.4%) (9,21). Retrospective studies showed that 19% of parotid gland and 79% of extraparotid gland tumors that were originally diagnosed as AciCC had been reclassified as SC/MASC (14). The site of origin for MASC/SC is the parotid gland in about two-thirds of reported cases; the other sites of origin are the intraoral minor salivary gland and the submandibular gland. Compared to AciCC, SC/MASC appears more often in non-parotid sites. Recent studies suggested that most non-parotid gland tumors diagnosed as AciCC might have a high probability of being reclassified as SC/MASC (13). SC/MASC has a higher incidence of regional lymph node involvement than AciCC (9,15). SC/MASC occurs in both children and adults (13 to 72 years), with an average age of 44.2 years (8-10), and has a slight male predilection, while AciCC predominantly affects women. Based on the previously reported cases, the overall male-to-female ratio was 1.5:1 (9).
Histologic and immunohistochemical features
Histologically, the cells of this tumor consist of the microcystic, tubular, solid, and papillary architecture, and characteristically have abundant extracellular material (1,5,17,22). These growth patterns of tumor cells overlap considerably between SC/MASC and AciCC. The cyst wall may contain a single cell lining, and the cells in these foci often show hobnailing (8,10). The cystic spaces often contain eosinophilic and periodic acid-Schiff staining-positive secretory material. Immunohistochemically, SC/MASC is characterized by strong S-100 protein, mammaglobin, vimentin, whereas AciCC has moderate or weak staining for S-100 protein and vimentin (8). Recent immunohistochemical analyses indicated that DOG1 staining was reliable for differential diagnosis. AciCC was reported to show diffuse DOG1 staining, whereas SC/MASC was negative or focal staining. Combined DOG1 and mammaglobin immunohistochemistry is reported comparable to ETV6-breakapart analysis for differentiating between papillary cystic variants of AciCC and SC/MASC (23-25) (Table 1). In addition, SC/MASC is known to express GATA3, pan-cytokeratin (AE1-AE3 and CAM5.2), CK7, CK8, CK18, CK19, epithelial membrane antigen (EMA), MUC1, MUC4, STAT5a, GCDFP15, adipophilin, etc., and is reported to be negative for calponin, smooth muscle actin (SMA), CK14, CK5/6, p63, etc. (8,26,27). Even with those immunohistochemical markers, some cases represent diagnostic difficulties. Zymogen-poor AciCC is difficult to be distinguished from SC/MASC without ETV6-breakapart analysis.
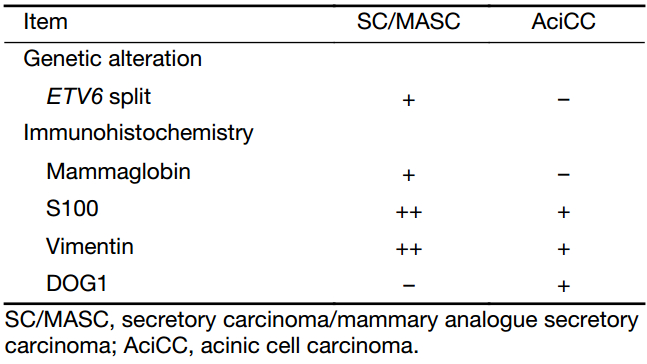
Full table
Gene translocations and gene fusions
Translocations are speculated to occur in about 20% of all cancers. In the salivary gland, four cancers have been reported harboring recurrent translocations (28,29). These include adenoid cystic carcinoma (AdCC), hyalinizing clear cell carcinoma (HCCC), MEC, and the newly identified SC (11,28) (Table 2).
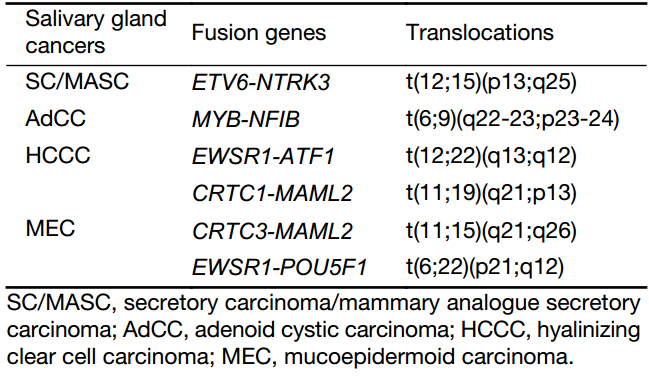
Full table
SC/MASC harbors a t(12;15)(p13;q25) translocation that results in an ETV6-NTRK3 fusion product (19). The ETV6-NTRK3 fusion gene encodes a chimeric tyrosine kinase, and this fusion results in a constitutively active chimeric tyrosine kinase mitogenic pathway and the PI3K-AKT pathway (6,7). This fusion occurred in more than 90% of SC/MASC cases (9,10,28). The fusion protein has transforming activity in multiple cell lineages and might have different effects in different organs, characteristics that are also described in infantile fibrosarcoma and acute myelogenous leukemias (22,30,31). ETV6-NTRK3 translocation has been identified by fluorescence in situ hybridization (FISH), reverse transcription-polymerase chain reaction (RT-PCR), or both. For FISH analysis, a dual-color break-apart probe for the ETV6 gene exhibits a split signal in the nuclei, which indicates that the ETV6 gene is not intact (19). In analysis utilizing RT-PCR to identify the ETV6 translocations, the 110-bp fusion transcript of the ETV6-NTRK3 can be detected (19).
In AdCC, a fusion of the myeloblastosis oncogene (MYB) to the transcription factor nuclear factor I/B (NFIB) has been identified approximately in one-third or one-half of cases of this carcinoma. It has been demonstrated that MYB was overexpressed in the majority of AdCCs, not only in those with MYB-NFIB fusions but also in those without the fusion. This suggests that MYB may be crucial in the pathogenesis of AdCC (32).
HCCC is a rare, low-grade tumor with a good prognosis. A disease-defining translocation in the Ewing sarcoma RNA-binding protein 1 (EWSR1) gene was identified in more than 80% of HCCCs (33). A t(12;22)(q13;q12) translocation produces the most common fusion transcript consisting of the genes EWSR1 and activating transcription factor 1 (ATF1) (28).
MEC harbors t(11;19)(q21;p13) translocation, which produces CRTC1-MAML2 and less frequently CRTC3-MAML2 t(11;15)(q21;q26) or EWSR1-POU5F1 t(6;22)(p21;q12) (9,11,15,29,34). With regard to MEC, the fusion-positive cases tend to have better outcomes, less recurrence, fewer metastases, and lower tumor-related mortality than fusion-negative cases (35). Thus, those salivary tumors are not typically high grade or morphologically heterogeneous.
In addition to the above cancers, pleomorphic adenoma, even though it is a benign mixed tumor, is known to have translocations involving PLGA1 or HMGA2 with a high probability (2,11,36).
Therapy for SC/MASC
SC/MASC often progresses slowly and is asymptomatic, and is generally regarded as a low-grade tumor. However, the clinical behavior of SC/MASC ranges from slowly growing tumors that infrequently recur after surgical resection to aggressive tumors that cause widespread metastasis and death. The initial choice of treatment is surgical resection, similarly to other salivary gland tumors. However, some patients with SC/MASC that is regarded as high-grade tumor received radiation therapy or chemoradiotherapy postoperatively (9,15). Although there was no statistically significant difference in the disease-free survival rate between SC/MASC and AciCC, some reports showed that the mean disease-free survival for patients with SC/MASC tended to be worse than that for patients with AciCC (8,9,15). Since the differences between SC/MASC and AciCC are slight in terms of clinicopathological features and outcome, the treatment for SC/MASC should follow those for AciCC. However, SC/MASC has a higher incidence of regional lymph node involvement and more aggressive behavior than AciCC (9,15). Most patients with SC/MASC appear to follow an indolent course, while certain cases appear predisposed to distant metastasis and increased mortality. ETV6-NTRK3 translocation might have contributed to the clinicopathological difference between SC/MASC and AciCC, therefore, testing for ETV6 translocation may be essential for the treatment of salivary gland tumors. In addition, ETV6 translocation may have value in treatment and may represent a therapeutic target in SC/MASC. ETV6 can fuse with other genes such as ABL1, RUNX1 or FLT3 (37-39). In leukemia, the fusion of the ETV6 occasionally responds to TK inhibitors (37,38,40). Additionally, in vitro studies of mammary SC showed that insulin-like growth factor receptor 1 (IGFR1) inhibitors might be useful to block ETV6-NTRK3 translocation-driven oncogenesis (6).
Conclusions
SC/MASC is a salivary gland tumor that is characterized by harboring ETV6-NTRK3 translocation. In the near future, the identification of translocations and/or gene fusions will be a critical factor in diagnosing salivary gland tumors and selecting appropriate treatments for them.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ozguroglu M, Tascilar K, Ilvan S, et al. Secretory carcinoma of the breast. Case report and review of the literature. Oncology 2005;68:263–8. [PubMed] DOI:10.1159/000086782
- Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J 2006;85:74. [PubMed]
- Brandt SM, Swistel AJ, Rosen PP. Secretory carcinoma in the axilla: probable origin from axillary skin appendage glands in a young girl. Am J Surg Pathol 2009;33:950–3. [PubMed] DOI:10.1097/PAS.0b013e31819c2628
- Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma . Cancer Cell 2002;2:367–76. [PubMed]
- Laé M, Fréneaux P, Sastre-Garau X, et al. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol 2009;22:291–8. [PubMed] DOI:10.1038/modpathol.2008.184
- Tognon CE, Somasiri AM, Evdokimova VE, et al. ETV6-NTRK3-mediated breast epithelial cell transformation is blocked by targeting the IGF1R signaling pathway. Cancer Res 2011;71:1060–70. [PubMed] DOI:10.1158/0008-5472.CAN-10-3096
- Wai DH, Knezevich SR, Lucas T, et al. The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells. Oncogene 2000;19:906–15. [PubMed] DOI:10.1038/sj.onc.1203396
- Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34:599–608. [PubMed] DOI:10.1097/PAS.0b013e3181d9efcc
- Chiosea SI, Griffith C, Assaad A, et al. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 2012;61:387–94. [PubMed] DOI:10.1111/j.1365-2559.2012.04232.x
- Connor A, Perez-Ordoñez B, Shago M, et al. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol 2012;36:27–34. [PubMed] DOI:10.1097/PAS.0b013e318231542a
- Weinreb I. Translocation-associated salivary gland tumors: a review and update. Adv Anat Pathol 2013;20:367–77. [PubMed] DOI:10.1097/PAP.0b013e3182a92cc3
- Bishop JA, Yonescu R, Batista DA, et al. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol 2013;121:228–33. [PubMed] DOI:10.1002/cncy.21245
- Bishop JA, Yonescu R, Batista D, et al. Most nonparotid " acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol 2013;37:1053–7. [PubMed] DOI:10.1097/PAS.0b013e3182841554
- Bishop JA. Unmasking MASC: bringing to light the unique morphologic, immunohistochemical and genetic features of the newly recognized mammary analogue secretory carcinoma of salivary glands. Head Neck Pathol 2013;7:35–9. [PubMed] DOI:10.1007/s12105-013-0429-0
- Chiosea SI, Griffith C, Assaad A, et al. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol 2012;36:343–50. [PubMed] DOI:10.1097/PAS.0b013e318242a5b0
- Griffith CC, Stelow EB, Saqi A, et al. The cytological features of mammary analogue secretory carcinoma: a series of 6 molecularly confirmed cases. Cancer Cytopathol 2013;121:234–41. [PubMed] DOI:10.1002/cncy.21249
- Levine P, Fried K, Krevitt LD, et al. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol 2014;42:49–53. [PubMed] DOI:10.1002/dc.22886
- Pisharodi L. Mammary analog secretory carcinoma of salivary gland: cytologic diagnosis and differential diagnosis of an unreported entity. Diagn Cytopathol 2013;41:239–41. [PubMed] DOI:10.1002/dc.21766
- Abe M, Inaki R, Kanno Y, et al. Molecular analysis of a mammary analog secretory carcinoma in the upper lip: Novel search for genetic and epigenetic abnormalities in MASC. Int J Surg Case Rep 2015;9:8–11. [PubMed] DOI:10.1016/j.ijscr.2015.02.011
- Khalele BA. Systematic review of mammary analog secretory carcinoma of salivary glands at 7 years after description. Head Neck 2017;39:1243–8. [PubMed] DOI:10.1002/hed.24755
- Pia-Foschini M, Reis-Filho JS, Eusebi V, et al. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol 2003;56:497–506. [PubMed]
- Knezevich SR, Garnett MJ, Pysher TJ, et al. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma . Cancer Res 1998;58:5046–48. [PubMed]
- Said-Al-Naief N, Carlos R, Vance GH, et al. Combined DOG1 and mammaglobin immunohisto-chemistry is comparable to ETV6-breakapart analysis for differentiating between papillary cystic variants of acinic cell carcinoma and mammary analogue secretory carcinoma. Int J Surg Pathol 2017;25:127–40. [PubMed] DOI:10.1177/1066896916670005
- Khurram SA, Sultan-Khan J, Atkey N, et al. Cytogenetic and immunohistochemical characterization of mammary analogue secretory carcinoma of salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:731–42. [PubMed] DOI:10.1016/j.oooo.2016.07.008
- Hsieh MS, Chou YH, Yeh SJ, et al. Papillary-cystic pattern is characteristic in mammary analogue secretory carcinomas but is rarely observed in acinic cell carcinomas of the salivary gland. Virchows Arch 2015;467:145–53. [PubMed] DOI:10.1007/s00428-015-1786-8
- Mariano FV, dos Santos HT, Azanero WD, et al. Mammary analogue secretory carcinoma of salivary glands is a lipid-rich tumour, and adipophilin can be valuable in its identification. Histopathology 2013;63:558–67. [PubMed] DOI:10.1111/his.12192
- Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol 2013;7 Suppl 1:S30–6. [PubMed] DOI:10.1007/s12105-013-0455-y
- Stenman G. Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol 2013;7 Suppl 1:S12–9. [PubMed] DOI:10.1007/s12105-013-0462-z
- Yin LX, Ha PK. Genetic alterations in salivary gland cancers. Cancer 2016;122:1822–31. [PubMed] DOI:10.1002/cncr.29890
- Bourgeois JM, Knezevich SR, Mathers JA, et al. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors . Am J Surg Pathol 2000;24:937–46. [PubMed]
- Kralik JM, Kranewitter W, Boesmueller H, et al. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn Pathol 2011;6:19. [PubMed] DOI:10.1186/1746-1596-6-19
- Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance . Clin Cancer Res 2010;16:4722–31. [PubMed] DOI:10.1158/1078-0432.CCR-10-0463
- Simpson RH, Skálová A, Di Palma S, et al. Recent advances in the diagnostic pathology of salivary carcinomas. Virchows Arch 2014;465:371–84. [PubMed] DOI:10.1007/s00428-014-1639-x
- Fehr A, Röser K, Heidorn K, et al. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes Chromosomes Cancer 2008;47:203–6. [PubMed] DOI:10.1002/gcc.20522
- Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer 2006;45:470–81. [PubMed] DOI:10.1002/gcc.20306
- Abe M, Mori Y, Kanno Y, et al. A case of pleomorphic adenoma of the parotid gland with multiple local recurrences through facial to cervical region. Open J Stomatol 2014;4:441–5. DOI:10.4236/ojst.2014.49059>
- Walz C, Erben P, Ritter M, et al. Response of ETV6-FLT3-positive myeloid/lymphoid neoplasm with eosinophilia to inhibitors of FMS-like tyrosine kinase 3 . Blood 2011;118:2239–42. [PubMed] DOI:10.1182/blood-2011-03-343426
- Wlodarska I, Mecucci C, Baens M, et al. ETV6 gene rearrangements in hematopoietic malignant disorders . Leuk Lymphoma 1996;23:287–95. [PubMed] DOI:10.3109/10428199609054831
- Jabber Al-Obaidi MS, Martineau M, Bennett CF, et al. ETV6/AML1 fusion by FISH in adult acute lymphoblastic leukemia. Leukemia 2002;16:669–74. [PubMed] DOI:10.1038/sj.leu.2402435
- O’Brien SG, Vieira SA, Connors S, et al. Transient response to imatinib mesylate (STI571) in a patient with the ETV6-ABL t(9;12) translocation . Blood 2002;99:3465–7. [PubMed]
