Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) has significant clinical relevance because it is a common side effect of some of the most widely used chemotherapy agents. CIPN may cause potentially dose-limiting side effects, thus impairing the effectiveness of treatment and the patients’ quality of life (QOL) (1).
Over the past decade, paclitaxel is a widely used chemotherapy drug for solid tumors, and has greatly influenced the standard of treatment in breast and ovarian carcinomas. Paclitaxel facilitates the polymerization of tubulin into highly stable, intracellular microtubules (2). As microtubules cause cell death by interfering with normal cell division, paclitaxel has become a significant component of cancer care for both early-stage disease and advanced disease.
Unfortunately, the paclitaxel chemotherapy regimen is associated with significant peripheral neurotoxicity as a side effect that may cause potential disability or even offset the therapeutic benefits (3). In particular, peripheral neuropathy can be a severe adverse effect, which can be a dose-limiting toxicity, and may lead to dose reduction or even cessation of therapy (4). Thus, if one is to gain the overall benefit from paclitaxel chemotherapy, we should balance the long-term toxicities along with the efficacy of treatment to improve the clinical endpoints such as response rate, time to treatment failure, and overall survival (5).
Although different clinical scales for cancer patients are currently available, an accurate grading of CIPN is still a controversial issue (particularly in clinical trials). National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE) was already formally validated in patients with cancer (6,7). This is mainly due to the lack of sensitivity of the proposed scales. Moreover, the grading may also differ between various examiners (7). In this study, we evaluated the peripheral neurotoxicity by using oncologic grading scales, namely the Functional Assessment of Cancer Therapy-Taxane (FACT-Tax) Scales (8).
Comparative information regarding duloxetine’s efficacy was already available from a series of randomized, double-blind, placebo-controlled, phase III clinical studies in diabetic populations, where prior treatment with other oral anti-neurotoxicity drugs yielded unsatisfactory results (9,10). However, there are insufficient data on duloxetine’s use in patients with CIPN, which may occur in a clinical oncology setting. The objective of our study was to determine the efficacy and safety of flexible-doseduloxetine in treating Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy (PIPN) (11).
Materials and methods
Description of scales
The FACT-Tax comprises the FACT-General (FACT-G) plus a specific paclitaxel subscale, including the neurotoxicity component items (Table 1). The high psychometric properties used by these evaluation criteria were previously described; this scale was used as a self-report evaluation instrument to measure the health-related QOL of patients receiving paclitaxel-regimen during a 12-week treatment course (3). This scale contains questions scored from 0 to 4 (0, not at all; 4, very much), and then the scores are summed (total score range, 0–44). Arguably, the FACT-Tax Scale could be more appropriate for investigating and accurately reporting the severity of CIPN than a five-grade scale. To overcome any discrepancy due to the dropouts during the treatment period, data from the “complete case” analyses are presented here.

Full table
In the absence of published data defining a cut-off point for determining a clinically significant change in the FACT-Tax score, we defined any clinically meaningful improvement as a declined score in the FACT-Tax measures. The FACT-Tax Scale was also calculated after stratification of the entire population into two subgroups. Furthermore, we also calculated the proportion of the patients experiencing any decrease in the FACT-Tax Scale score (3).
Study participants
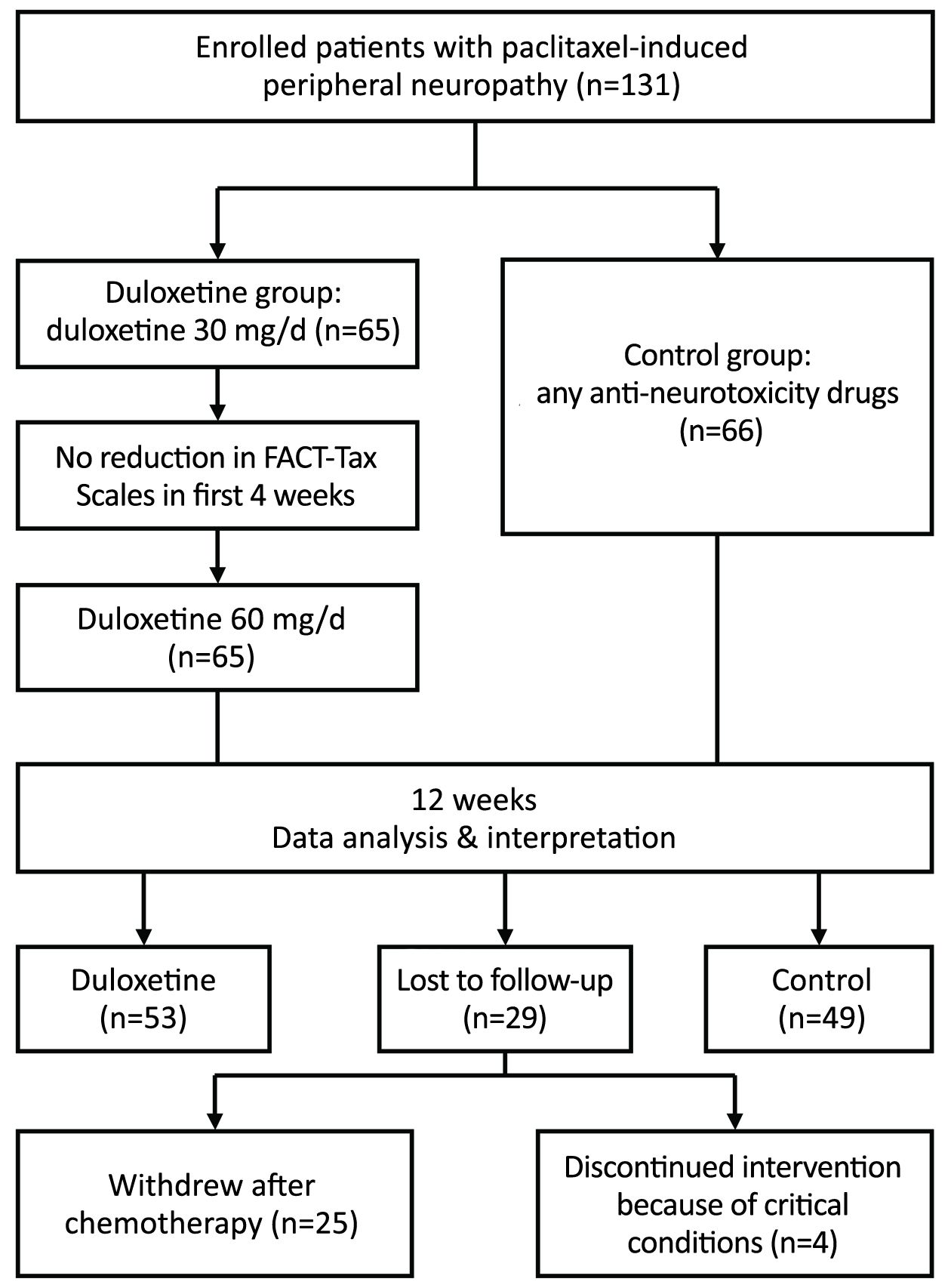
The flexible-dose, open-label, single-center, prospective study was conducted from November 2014 to January 2017. A total of 131 breast cancer patients were enrolled into this study after surgical excision of the tumor, according to the inclusion criteria set as follows: 1) female; 2) age from 25 to 65 years; 3) satisfactory liver and kidney function; 4) Eastern Cooperative Oncology Group (ECOG) scores of 0 or 1; 5) treatment with courses of 175 mg/m2 paclitaxel regimens; and 6) sensory neuropathy as evaluated by the National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE). In summary, 29 patients were lost to follow up: 4 patients discontinued the study due to critical health conditions and 25 patients were unwilling to continue with this study. Thus, a total of 102 patients (77.9%) completed the study as planned, with a median age of 50 (range, 25–60) years. Several factors that predisposed the patients to CIPN before the study were excluded, including those with CIPN associated diseases (e.g., hepatitis B virus infection status, diabetes mellitus, and thyroid dysfunction), with existing alcohol abuse, requiring a nutritional supplement, or with a previous history of chemotherapy.
Intervention
The eligible patients were classified into two groups. The duloxetine group (n=53) received duloxetine during the initial first 4 weeks of treatment and then for an additional 8 weeks, and the control group (n=49) received any other anti-neurotoxicity therapy, such as vitamin B, fish oil and nonsteroidal anti-inflammatory agents during the same crossover period (Figure 1).
Data collection and instruments
Patient-reported peripheral neurotoxic severity and functional abnormality were assessed by the FACT-Tax Scales containing 11 questions. Items were scored from 0 to 4 (0, not at all; 4, very much) and subsequently the scores were summed up (total score range, 0–44) (Table 1).
Ethics statement
This study protocol was approved by the Independent Ethics Committee and Institutional Review Board of the National Cancer Center, China. All the research was conducted in compliance with the ethical principles and the regulatory guidelines in accordance with Good Clinical Practice requirements. All the enrolled patients provided written informed consent before their treatment.
Statistical analysis
The efficacy evaluation was conducted using the full analysis set, which included all the patients who received at least one dose of duloxetine or any other anti-neurotoxicity treatment. The specific safety analysis set included only those patients who received at least one dose of duloxetine.
All the available data from the complete cases were used for the calculation of changed scores over time by subtracting the baseline scores at subsequent assessments. We use description of categorical variables and Chi-square test for equilibrium between baseline data. Multifactor logistic regression was used to test FACT-Tax decrease in the duloxetine group and control group in IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA). Risks were reported as odds ratios (OR) along with their 95% confidence intervals (95% CI). FACT-Tax score improvement should be distributed according to the characteristics of the data. The data do not conform to the normal distribution, so we use median and inter-quartile range (25% digits, 75% digits) to represent the statistical results and Mann-Whitney U test is used for comparison. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Study participants’ clinical characteristics
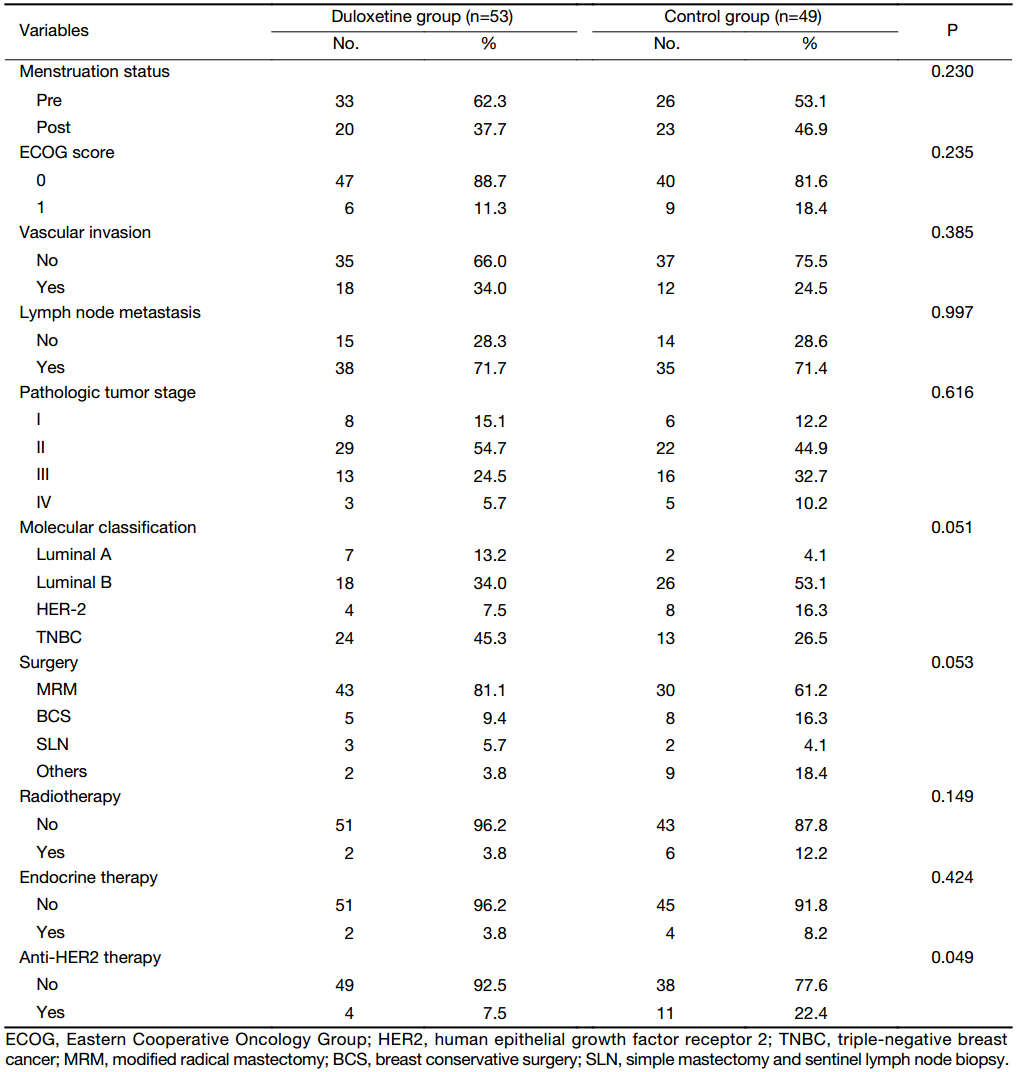
A total of 102 female breast cancer patients completed the treatment. Both the groups were well balanced during the enrollment phase of this study. Each patient received 4 or 6 cycles of adjuvant chemotherapy containing paclitaxel regimens, administered intravenously every 2 or 3 weeks. Radiation therapy (as indicated) and endocrine therapy, for patients with hormone receptor-positive disease, were administered after the completion of chemotherapy (Table 2). They were classified into either the duloxetine group (n=53) or the control group (n=49). The mean age was 47.9±7.8 years in the duloxetine group and 49.6±9.7 years in the control group (P=0.198). In addition, there was no statistically significant difference in the baseline between these two groups (Table 2).
Full table
Primary efficacy
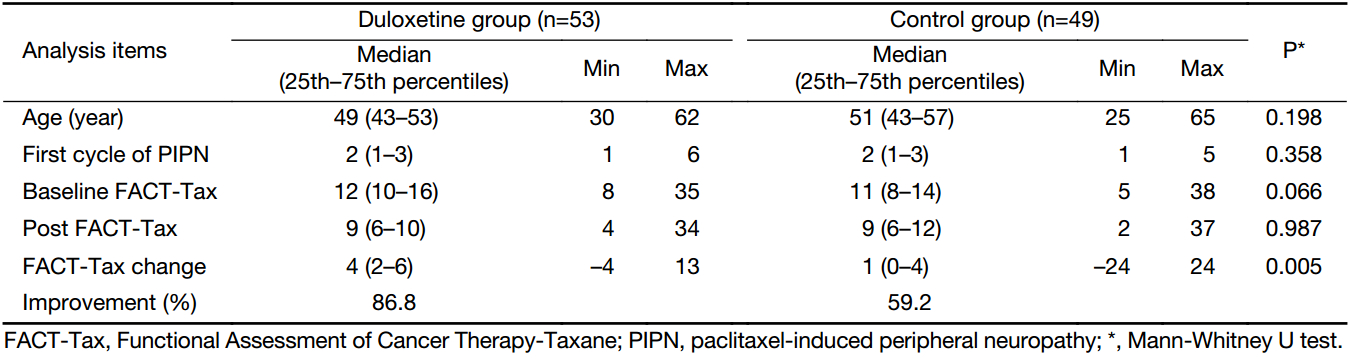
The changes in the severity of neuropathic pain were evaluated by using the FACT-Tax assessments. Of the remaining 102 patients, 75 patients (73.5%) had an improvement in their FACT-Tax scores. The “reduced FACT-Tax score” of the 53 patients in the duloxetine group was as follows: 7 patients (13.2%) did not develop PIPN, and 46 patients (86.8%) manifested some reduction in their neurotoxicity scores. In the control group, the degraded FACT-Tax score was not observed in 20 patients (40.8%), while 29 patients (59.2%) developed some PIPN improvement. Between the duloxetine group and the control group, a significant difference was observed in the degree of decrease in the severity of PIPN (OR=5.426; 95% CI, 1.898–15.514; P=0.002). The median (25th–75th percentiles) decrease of FACT-Tax pain score in the duloxetine group and the control group was 4 (2–6) vs. 1 (0–4) (P=0.005) (Table 3).
Full table
Safety
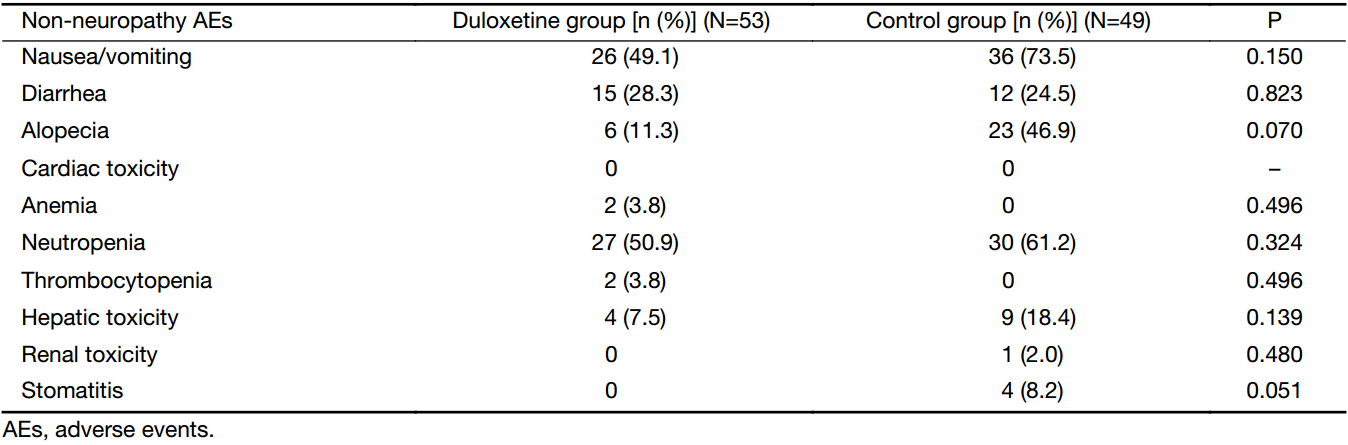
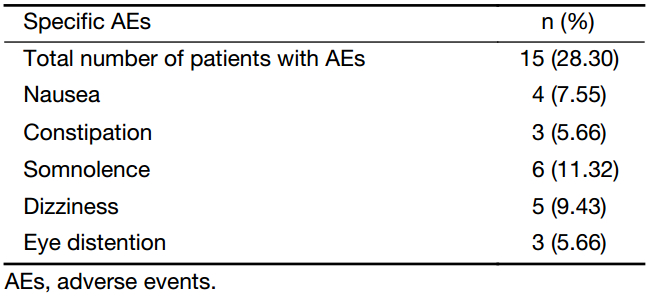
We found in our study that treating PIPN with duloxetine did not interfere with chemotherapy. Non-neuropathy adverse events that are attributed to chemotherapy were mild and similar in both groups. No significant differences were observed in the incidence of PIPN occurring in patients who were being treated with duloxetine or the other anti-neurotoxicity drugs. The incidence data for grade 3 or 4 non-neuropathy chemotherapy adverse effects are presented in Table 4. Among them, safety findings were consistent with the known duloxetine safety profile in treating chronic PIPN with tolerable toxicity at a dose of 60 mg/d. The major toxicities included nausea, constipation, somnolence, dizziness, and eye distention (Table 5).
Full table
Full table
Discussion
With the advancement of socio-economic factors, the incidence and mortality of breast cancer have increased in China (12-14). The overall survival rate of breast cancer patients has improved in recent years mainly due to the significant advances made in the field of medicine, especially in the development of novel chemotherapy strategies. However, the peripheral neuropathy caused by paclitaxel regimens not only decreases the patients’ QOL but also often forces the doctors to limit the chemotherapy dose or even interrupt the treatment (15). Identifying novel neuroprotective agents is vital since only a very few pharmacological options are currently available to effectively treat PIPN-related symptoms (16). Most anti-neurotoxicity drugs are tested for their short and inadequate pain relief. Thus, finding new, well-tolerated oral agents for effective remedy of PIPN has remained a challenge for oncologists (17,18). The currently available options to treat PIPN are as follows: tricyclic antidepressants (i.e. nortriptyline and amitriptyline) that play an important role in the treatment of neuropathy; however, there are limited data to prove their therapeutic benefit in PIPN. Analgesics (i.e., opioids or nonsteroidal anti-inflammatory agents) are only modestly effective in treating neuropathy, and they do not have long-term efficacy (19). In the absence of any standard therapeutic agent to prevent or treat chemotherapy-induced neuropathy, clinicians have often resorted to nutritional supplements (vitamins B, omega-3 fatty acids, and some minerals) (4).
Interestingly, duloxetine was previously found to be potent in treating peripheral neuropathic pain in diabetic patients, and is currently approved for such indications in several countries (9). The safety and efficacy of taking duloxetine at 60 mg or 120 mg per day were evaluated in a randomized double-blind, placebo-controlled study for the treatment of diabetic neuropathic pain syndromes (11,20). In June 2008, the United States Food and Drug Administration (FDA) licensed duloxetine as the first 5-serotonin and norepinephrine reuptake inhibitors for the treatment of fibromyalgia (21).
This study aimed to test the efficacy of duloxetine in treating PIPN. The enrolled breast cancer patients were assigned to take oral supplements of duloxetine or any anti-neurotoxicity drug during their crossover therapy period. Other factors need to be considered when judging the clinical significance include the drug’s safety, and other endpoints such as function and tolerability.
In our current study, two patient groups were well balanced at the time of research entry. Among the patients with PIPN, the use of duloxetine, when compared with any other anti-neurotoxicity treatment, resulted in an improvement in the FACT-Tax score. During the initial treatment, individuals receiving duloxetine treatment reported a mean decrease in the pain score (3.89 vs. 2.10) compared to the other anti-neurotoxicity drug treated group. The observed median FACT-Tax pain score change between the duloxetine group and the control group was 4.00 vs. 1.00, which was larger than the results observed in patients receiving duloxetine for US FDA approved indications for diabetic peripheral neuropathy and fibromyalgia pain (9,11). Between the duloxetine supplemented group and the control group, a significant difference was observed in decreasing PIPN (OR=5.426; 95% CI, 1.898–15.514; P=0.002).
Duloxetine-treated PIPN patients reported global improvement in their symptoms and functionality. Duloxetine was remarkably well-tolerated in this breast cancer population. Based on previous studies that evaluated duloxetine for different indications, no increase in the incidence of severe adverse effects was expected in this study with duloxetine use. Our safety findings were consistent with the reported duloxetine safety profile. Such toxicity was also not observed in other peripheral neuropathy trials (22). The incidence of nausea, constipation, somnolence and dizziness, as reported by those treated with duloxetine in the current study, was similar to previous reports. In our experience, swallowing the capsule dosage-form of duloxetine after meals, without chewing and crushing, could reduce the adverse outcome of nausea and vomiting. However, three cases of eye distention were found with duloxetine treatment in our study.
The observed mean difference in FACT-Tax pain score between the duloxetine and any other anti-neurotoxicity groups in paclitaxel chemotherapy regimens was larger than that reported by Goldstein (23). Duloxetine-related clinically meaningful improvement in other PIPN symptoms, such as weakness, joint pain, or muscle cramps, may be directly comparable with painful chemotherapy-induced peripheral neuropathy.
Conclusions
The findings from our study suggest that there is a significant benefit in using duloxetine to treat PIPN-related symptoms. FACT-Tax scores measured serially in this study reveal a statistically significant decline in the severity of PIPN-related pain in duloxetine-treated patients when compared to those treated with any other anti-neurotoxicity drugs. Long-term cancer pain, including PIPN, is highly prevalent among patients undergoing taxane-related chemotherapy for breast cancer. Management of moderate/severe anxiety and depression needs to be improved (24). Since the duloxetine-related clinical study is currently being carried out for the treatment of various chronic pain management, including osteoarthritis and chronic lumbago, duloxetine may be more effective in treating PIPN. Future clinical use is expected to be further studied, as the market potential is enormous and it is worthy of the clinical attention (25). Duloxetine is safe, tolerable, and promising at a dose of 60 mg/d in Chinese breast cancer patients with PIPN. Future research should examine duloxetine as a potential aid in the administration of anti-PIPN therapies.
Acknowledgements
We gratefully acknowledged the cooperation of all population-based registries in National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, in providing statistics and data collection. The authors assume full responsibility for database creation, analyses and interpretations of these data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008;44:1507–15. [PubMed] DOI:10.1016/j.ejca.2008.04.018
- Argyriou AA, Koltzenburg M, Polychronopoulos P, et al. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 2008;66:218–28. [PubMed] DOI:10.1016/j.critrevonc.2008.01.008
- Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: the functional assessment of cancer therapy-taxane (FACT-taxane). Cancer 2003;98:822–31. [PubMed] DOI:10.1002/cncr.11578
- Gutiérrez-Gutiérrez G, Sereno M, Miralles A, et al. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol 2010;12:81–91. [PubMed] DOI:10.1007/S12094-010-0474-z
- Smith EM, Cohen JA, Pett MA, et al. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs 2010;33:173–83. [PubMed] DOI:10.1097/NCC.0b013e3181c989a3
- Vasquez S, Guidon M, McHugh E, et al. Chemotherapy induced peripheral neuropathy: the modified total neuropathy score in clinical practice. Ir J Med Sci 2014;183:53–8. [PubMed] DOI:10.1007/s11845-013-0971-5
- Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–39. [PubMed] DOI:10.1016/j.jaad.2012.02.010
- Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology 2003;61:1297–300. [PubMed]
- Sultan A, Gaskell H, Derry S, et al. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol 2008;8:29. [PubMed] DOI:10.1186/1471-2377-8-29
- Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: A systematic review. Clin J Pain 2016;32:1005–1010. [PubMed] DOI:10.1097/AJP.0000000000000343
- Irving G, Tanenberg RJ, Raskin J, et al. Comparative safety and tolerability of duloxetine vs. pregabalin vs. duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract 2014;68:1130–40. [PubMed] DOI:10.1111/ijcp.12452
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12.
- Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1–11. [PubMed] DOI:10.3978/j.issn.1000-9604.2016.02.08
- Yan X, Han R, Zhou J, et al. Incidence, mortality and survival of female breast cancer during 2003-2011 in Jiangsu province, China. Chin J Cancer Res 2016;23:321–9.
- Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm 2014;71:19–25. [PubMed] DOI:10.2146/ajhp130126
- Reeves BN, Dakhil SR, Sloan JA, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer 2012;118:5171–8. [PubMed] DOI:10.1002/cncr.27489
- Postma TJ, Reijneveld JC, Heimans JJ. Prevention of chemotherapy-induced peripheral neuropathy: a matter of personalized treatment?. Ann Oncol 2013;24:1424–6. [PubMed] DOI:10.1093/annonc/mdt173
- Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord 2014;15:76. [PubMed] DOI:10.1186/1471-2474-15-76
- Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 2007;110:2110–8. [PubMed] DOI:10.1002/cncr.23008
- Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6:346–56. [PubMed] DOI:10.1111/j.1526-4637.2005.00061.x
- Murakami M, Osada K, Mizuno H, et al. A randomized, double-blind, placebo-controlled phase III trial of duloxetine in Japanese fibromyalgia patients. Arthritis Res Ther 2015;17:224. [PubMed] DOI:10.1186/s13075-015-0718-y
- Takenaka M, Iida H, Matsumoto S, et al. Successful treatment by adding duloxetine to pregabalin for peripheral neuropathy induced by paclitaxel. Am J Hosp Palliat Care 2013;30:734–6. [PubMed] DOI:10.1177/1049909112463416
- Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116:109–18. [PubMed] DOI:10.1016/j.pain.2005.03.029
- Valeberg BT, Miaskowski C, Paul SM, et al. Comparison of oncology patients’ and their family caregivers’ attitudes and concerns toward pain and pain management. Cancer Nurs 2016;39:328–34. [PubMed] DOI:10.1097/NCC.0000000000000319
- Carlos F, Espejel L, Novick D, et al. Duloxetine for the treatment of painful diabetic peripheral neuropathy in Venezuela: economic evaluation. Medwave 2015;15:e6265. [PubMed] DOI:10.5867/medwave.2015.08.6265