Management of extramedullary plasmacytoma: Role of radiotherapy and prognostic factor analysis in 55 patients
Introduction
Plasmacytomas originate from the monoclonal malignant transformation of plasma cells. These tumors are a type of B-cell non-Hodgkin lymphoma and encompass a group of neoplasms at different stages of maturity, including multiple myeloma (MM) and solitary plasmacytomas. The latter can be classified into two clinical subsets: solitary plasmacytoma of bone (SPB) and extramedullary plasmacytoma (EMP) (1,2). Although SPB and EMP originate from the same cell type and are initially restricted to a single area, the former tends to evolve into MM more frequently than the latter, and for this reason, the two diseases are often considered different pathologic entities (3,4).
EMP is a rare disease that represents approximately 3% of all plasma cell neoplasms and has a low incidence of 0.04 cases per 100,000 individuals around the world (2,5,6). The disease may originate in various soft tissues throughout the body, but more than 80% of these tumors arise in the head and neck (H&N), especially the upper aerodigestive tract (7,8).
At present, oncologists are still confused about how to choose the optimal therapeutic strategy for EMP patients due to the scarcity of published evidence. EMP responds well to local therapy, and surgery can achieve high local control (LC) rates of EMP in certain situations. However, radical excision is often difficult due to the size of the tumor and the proximity of vital organs, which may cause the disfigurement of some organs, especially those in the H&N (9). In addition, EMP is radiosensitive, and radiotherapy (RT) can elicit beneficial outcomes (6-8,10). Therefore, RT is recognized as the mainstay treatment for EMP. However, RT-resistant H&N EMP has been reported in some reports (11,12). Furthermore, the role of chemotherapy (CT) in treatment to reduce recurrence rates or to improve survival outcomes remains unclear. Some studies suggest that CT is not beneficial in the treatment of primary tumors but may be selected when RT is ineffective or upon tumor recurrence (4,13).
Due to the rarity of EMP and its long natural history, most studies on this subject are retrospective and include small series of patients. Thus, the prognostic factors are not well established, and the dose of RT necessary to achieve favourable LC remains controversial. The total doses recommended by previous investigators range from 35 Gy to 60 Gy (4,10,13).
The purpose of this retrospective study was to investigate potential prognostic factors affecting the outcomes of EMP and to evaluate the optimal method and effects of RT in the management of EMP.
Materials and methods
Patient characteristics
Between November 1999 and August 2015, the clinical data of 55 patients with pathologically proven diagnoses of EMP from Peking University Cancer Hospital and Sun Yat-sen University Cancer Center were reviewed. This study was approved by both institutional review boards.
All patients met the following diagnostic criteria: 1) one solitary lesion confirmed by a tissue biopsy (fine-needle or open) revealing plasma cell histology; 2) the absence of skeletal lytic lesions or other tissue involvement verified by imaging examination; 3) bone marrow aspirate/biopsy specimen with less than 5% plasma cells; and 4) no anaemia, hypercalcaemia or renal impairment due to plasma cell dyscrasia (9,14). After treatment, the patients were followed up by out-patient re-examination, phone calls and correspondence. Clinical symptoms were registered and imaging assessment data were collected to evaluate the recurrence of metastasis or progress to MM, and the survival data of the patients were also assessed. The follow-up deadline was January 31, 2016. The median follow-up period was 56 (range, 5–177) months.
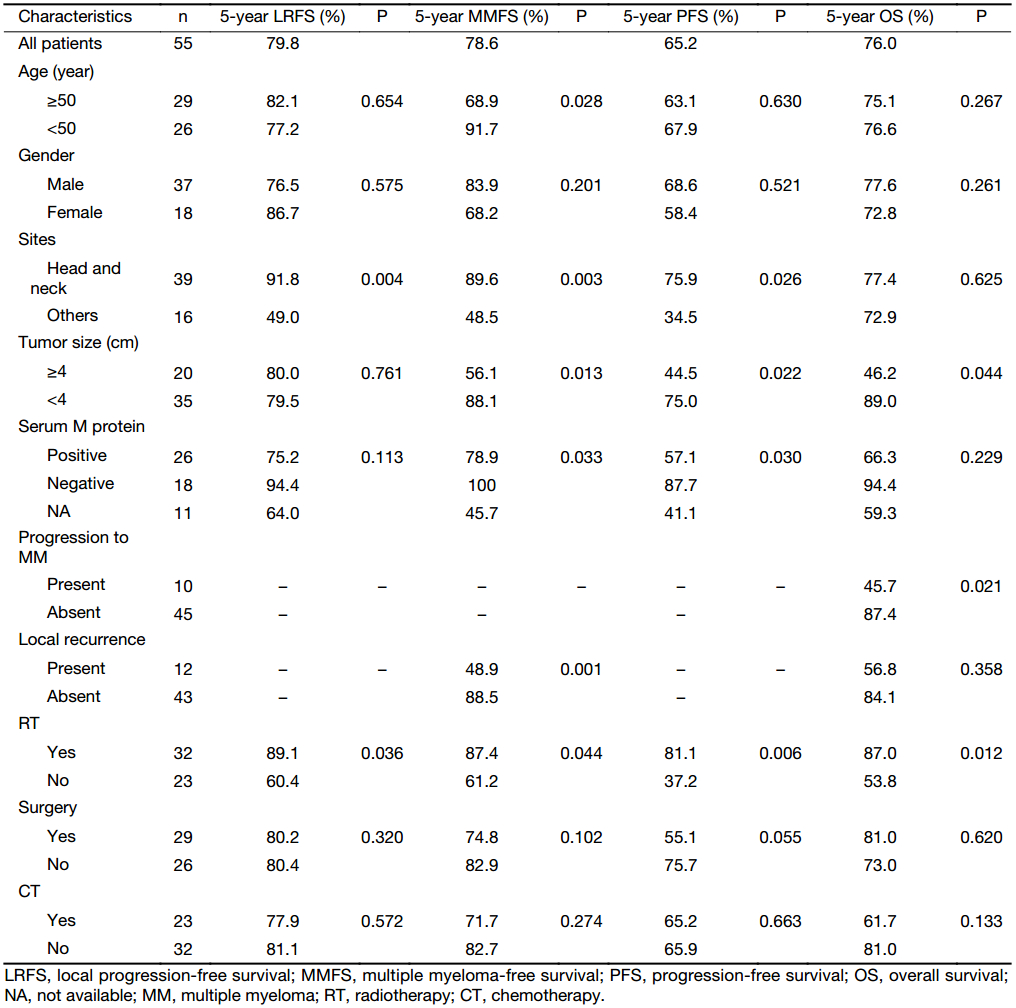
The median age at diagnosis was 51 (range, 22–77) years. Thirty-seven patients were male, and 18 were female. The median tumor size was 3.5 (range, 1.0–15.0) cm. Thirty-nine patients (70.9%) presented with disease in the head or neck region. Serum M protein was present in 26 of 44 patients (59.1%), and Bence Jones protein was present in 4 patients. The clinical characteristics of the patients are summarized in Table 1.
Full table
Treatment
Twelve patients were treated with definitive RT alone, 9 received surgery (S) alone, 3 received CT alone, and 3 patients did not receive any treatment. The remaining patients were treated with combination therapy, including 8 patients who received S+RT, 8 who received S+CT, 8 who received CT+RT, and 4 who received S+CT+RT.
RT was applied using linear accelerators with megavoltage beams (6 MV X-ray). The planning target volumes included the radiographically visible gross tumor and positive cervical nodes with a sufficient margin. Among the patients with H&N EMP, elective nodal irradiation (ENI) was performed in 8 patients with positive regional lymph nodes, including 5 patients who received whole-neck irradiation and 3 patients who received partial neck irradiation. Two-dimensional planning (15 patients), computed tomography simulation-based three-dimensional conformal radiotherapy (3D-CRT) planning (4 patients), and intensity-modulated radiotherapy (IMRT) planning (13 patients) were used. 3D-CRT and IMRT were planned using the Pinnacle3 system (Philips Healthcare, Andover, MA, USA).
The median applied dose was 50.0 (range, 30.0–70.0) Gy. The median single-fraction dose was 2.0 (range, 1.8–2.5) Gy.
Combined CT was administered to 23 patients (41.8%) for a median of 4 (range, 1–8) cycles, including vincristine, adriamycin and dexamethasone (VAD) in 6 patients, cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) in 5 patients, melphalan and prednisolone (MP), cyclophosphamide, vincristine, adriamycin and dexamethasone (CVAD), thalidomide and dexamethasone (TD), and bortezomib in 2 patients each and other combinations in 4 patients.
Statistical analysis
The examined endpoints included local recurrence-free survival (LRFS), multiple myeloma-free survival (MMFS), progression-free survival (PFS) and overall survival (OS). LRFS was calculated from the date of diagnosis to the date of local relapse. MMFS was calculated from the date of diagnosis until the date of progression to MM. The PFS was calculated from the date of diagnosis to the date of plasmacytoma progression, progression to MM, death due to any cause, or the last follow-up. The OS was calculated from the date of diagnosis to the time of death due to any cause or until the last follow-up.
The SPSS package (Version 16.0; SPSS Inc., Chicago, IL, USA) was used to establish the database. The survival curves were calculated using the Kaplan-Meier method and compared with log-rank tests. The Chi-square tests (Fisher’s exact test) were used to compare the distributions of acute radiation toxicities among the dose <45 Gy and ≥45 Gy groups. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Follow-up
At the time of last follow-up, local recurrence had developed in 12 patients (21.8%). The 5-year LRFS was 79.8%. The median time to local recurrence was 8.4 (mean, 24.6) months. Progression to MM was observed in 10 patients (18.2%). The 5-year MMFS was 78.6%. The median time from diagnosis to MM was 26.6 (mean, 29.1) months. The 5-year PFS was 65.2%. Twelve patients died (10 deaths were myeloma related, and 2 patients died of unrelated causes), and the 5-year OS was 76.0%.
Seven patients showed only local recurrence. Among these patients, one died due to uncontrolled disease, and 4 patients ultimately progressed to MM, of which 2 died due to the disease. Of the other 5 patients who exhibited both local and distant disease, 2 were successfully treated (one is alive without disease, and the other died of unrelated causes); the other 3 patients progressed to MM, and one died of the disease. Of the 43 remaining patients, 2 of the 3 patients who progressed to MM died of the disease, and among the patients who exhibited no signs of MM, 4 deaths were myeloma-related, and one patient died of unrelated causes.
Survival analysis
According to the univariate analyses (Table 1), RT was a favourable factor for all examined endpoints. Tumor located in the H&N had a favourable LRFS. Furthermore, the other factors associated with a favorable MMFS and PFS were age <50 years, tumor located in the H&N, tumor size <4 cm, and serum M protein negativity. In addition, the presentation of local recurrence was associated with poor MMFS. The factors that predicted better OS included tumor size <4 cm and the absence of progression to MM.
Analysis stratified by treatment modalities
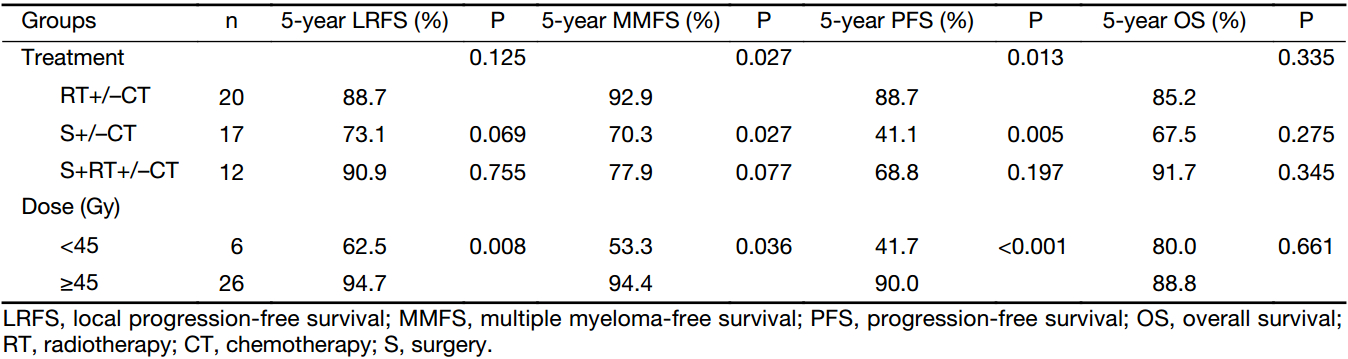
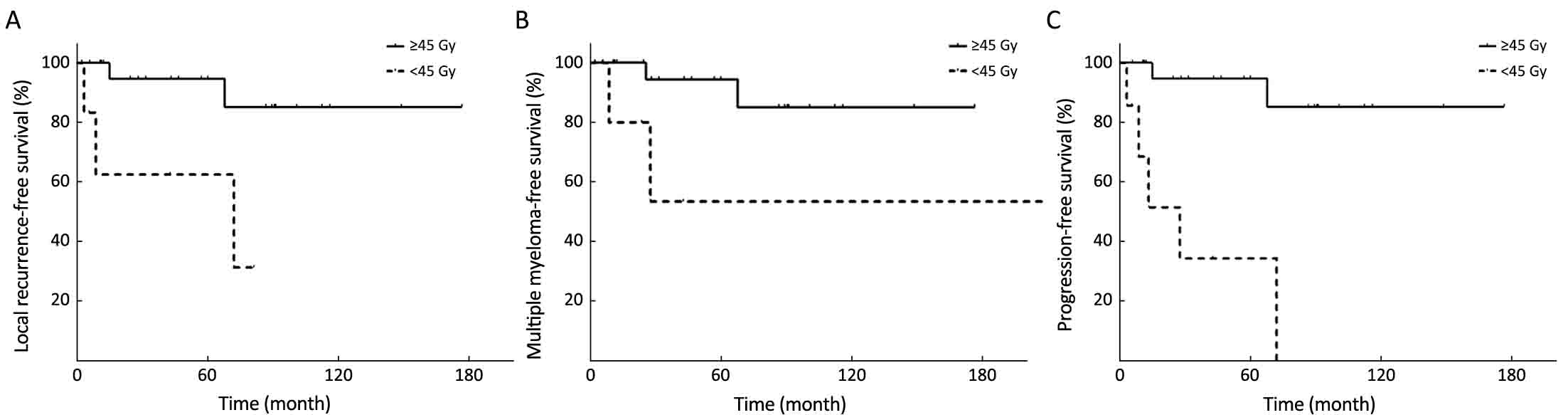
To explore the potential differences between the various treatment modalities, stratified analysis was adopted (Table 2). Univariate analysis of the treatment modalities revealed that compared with S+/–CT, RT+/–CT was a significantly favourable prognostic factor for MMFS and PFS and had a trend towards improved LRFS. Although there was no significant difference, S+RT+/–CT also had more favourable outcomes compared with S+/–CT. Among the patients who received RT, the ≥45 Gy group had more favourable 5-year LRFS, MMFS and PFS (Figure 1).
Full table
In patients with H&N EMP, the RT+/–CT group showed a lower local recurrence rate (15.8%, 3/19) than the S+/–CT group (62.5%, 5/8, P=0.015). In addition, the patients with regional disease who received ENI, including whole-neck irradiation in 5 and partial-neck irradiation in 3, had no local recurrence. Nevertheless, the other 11 patients, who did not receive ENI, also had no regional failure, although three patients experienced recurrence within the irradiated fields, including one patient who received 50 Gy RT+CT for an 8.0 cm tumor, one who received 40 Gy RT alone for a 1.0 cm tumor and one who received 36 Gy RT+CT for a 6.5 cm tumor.
Of the patients who received S+RT+/–CT, ten patients who received doses ≥45 Gy had no local recurrence. Of the two patients who received doses <45 Gy, one patient with non-H&N EMP who received postoperative RT with 39.4 Gy for macroscopic disease developed a recurrence within the irradiated fields, and the other one, who had a negative margin, had no local recurrence after postoperative RT with 40 Gy.
Toxicities associated with CT
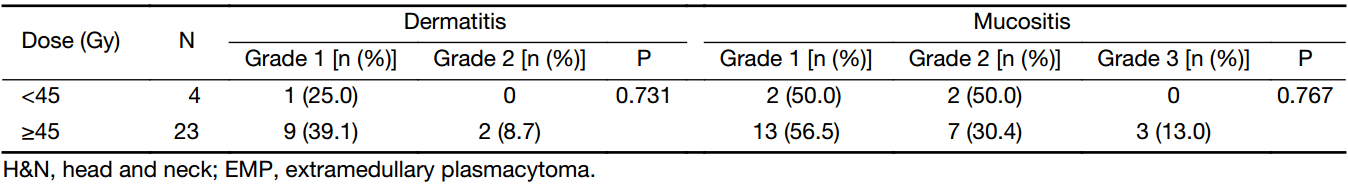
Acute radiation toxicities were examined according to the Common Toxicity Criteria for Adverse Events (version 4.0). The patients who received radiation of the H&N mainly suffered from mild acute dermatitis and mucositis, and three patients with EMP involving the nasopharynx experienced grade 3 radiation mucositis in the dose ≥45 Gy group (Table 3). Among patients with non-H&N EMP, grade 1 radiation dermatitis was obtained from one patient (1/2) in the dose <45 Gy group and one patient (1/3) in the dose ≥45 Gy group. No grade 4 and 5 acute or late radiation toxicity was recorded. In general, local RT was well-tolerated.
Full table
Discussion
In the present study, EMP was more common in males; the ratio of males to females was 2.1:1.0. The median age was 51 years. The majority of patients (70.9%) presented with disease in the head or neck regions and had a favourable prognosis. These results are similar to the data reported by others (3,12-14).
Prognostic factors that influence the outcomes of EMP patients have been reported in several series and include age, tumor size, serum M protein, and therapeutic approach (10,13,15,16). The majority of previous studies have compared EMP with SPB, and only a few studies have performed meaningful comparisons of H&N EMP with those at other sites. Alexiou et al. (5) analyzed 721 EMP cases and found no differences in recurrence (22.0% vs. 21.2%) or conversion to MM (16.1% vs. 14.1%) between EMPs of the upper aerodigestive tract and those occurring at other sites. However, a large retrospective multicenter study consisting of 258 EMP patients demonstrated that EMPs in the H&N were more closely associated with favourable PFS than those in other sites (median PFS times, 7.4 years vs. 3.1 years, P=0.025) (17). According to Gerry et al. (18), EMP of the H&N should be regarded as a unique pathologic entity with significantly higher 5-year disease-specific survival and OS than other plasmacytomas (P<0.001). However, these authors were unable to obtain LRFS and PFS data. In contrast, our results demonstrated that EMP located in the H&N exhibited superior 5-year LRFS, MMFS and PFS rates compared with non-H&N EMP, but no difference in OS was observed.
Tumor size is an important prognostic factor for outcome, although other studies have not found tumor size to be related to prognosis (19,20). Tsang et al. (10) reported on 46 patients with solitary plasmacytomas and found that the patients with tumors <5 cm exhibited a better 8-year LC rate than those with larger tumors (100% vs. 38%, P<0.010). Moreover, Zhu et al. (21) suggested that patients with tumors ≥5 cm had significantly poorer OS (P=0.001) and disease-free survival (DFS) (P<0.001) compared with those with tumors <5 cm. Additionally, a European multicentre retrospective study found that the 10-year OS was greater for tumors measuring <4 cm (72%) than for those ≥4 cm (61%, P<0.001) (17). In the current study, although tumor size was not related to LRFS, we observed that the patients with tumors <4 cm exhibited better 5-year MMFS (88.1% vs. 56.1%, P=0.013), 5-year PFS (75.0% vs. 44.5%, P=0.022), and 5-year OS rates (89.0% vs. 46.2%, P=0.044) than the patients with tumors ≥4 cm.
Positivity for plasma serum M protein at diagnosis is a prognostic factor for disseminated disease. Tournier-Rangeard et al. (12) reported on 17 patients with H&N EMP and found that the patients with positive for serum M protein at diagnosis exhibited poorer 5-year DFS (16.7% vs. 90.9%, P=0.008) and 5-year MMFS rates (33% vs. 100%, P=0.016). Other studies also reported that the presence of M protein indicated a higher incidence of conversion to MM (22,23). In the present series, serum M protein was present at diagnosis in 26 (47.3%) of 55 patients. The patients with initial serum M protein exhibited a poorer 5-year PFS rate (57.1% vs. 87.7%, P=0.030) and a higher risk of progression to MM (P=0.033).
Progression to MM also remains the main problem associated with EMP, especially for older patients. Some previous studies found that advanced age is associated with a higher risk of progression to MM but does not influence LC (10,17). Our study identified that progression to MM occurred more frequently in older patients, with patients <50 years old showing better 5-year MMFS (91.7% vs. 68.9%, P=0.028) than those ≥50 years old, and found that local recurrence after treatment was associated with the risk of progression to MM (P=0.001). Furthermore, the current study clearly demonstrated that patients without progression to MM had a significantly better 5-year OS rate (87.4% vs. 45.7%, P=0.021). In addition, patients with local recurrence had a worse OS (56.8% vs. 84.1%, P=0.358). There was no statistically significant difference, possibly due to the small number of patients.
EMP is well known to be radiation sensitive, and several investigators have demonstrated that high LC rates of 85%–100% can be achieved with adequate doses of RT (2,8,14,15,24). In our series, the LRFS of the patients who received RT was 89.1% at 5 years, which is consistent with previous reports. Our results also demonstrated that the patients who received RT exhibited superior 5-year MMFS, PFS and OS.
Alexiou et al. (5) recommended surgery followed by RT for EMP when complete resection is difficult to achieve. Bachar et al. (2) reported on 68 patients with H&N EMP and found that the local recurrence, regional recurrence and progression to MM rates following RT alone were 12.8% (5/39), 5.1% (2/39), and 17.9% (7/39), respectively, and the corresponding values were 12.5% (1/8), 25.0% (2/8), and 50.0% (4/8) following surgery alone. They also found that surgery without RT decreased the 5-year local recurrence-free rate from 82% to 75%. These authors thus recommended that RT should be considered as the primary therapy and that postoperative RT should be applied for patients with involved surgical margins but is not necessary for those who have undergone complete surgical excision with negative margins. However, in the report of Ozsahin et al. (17), nine (3%) patients underwent complete resection with negative margins, and only one of them received postoperative RT; subsequently, 7 patients relapsed. These findings argue against surgery alone, even in cases in which complete resection with negative margins was achieved. Suh et al. (25) suggested that RT alone can achieve a high LC rate and suggested that a large number of patients may not require surgery except when moderate doses of RT are challenging due to adjacent normal critical organs.
In our series, the RT+/–CT group exhibited significantly better 5-year PFS and MMFS rates than the S+/–CT group. Among the patients with H&N EMP, the RT+/–CT group showed a lower local recurrence rate (15.8%) than the S+/–CT group (62.5%). Thus, our results suggest that RT is curative in the majority of patients with EMP and that surgery without RT is not sufficient, particularly for H&N EMP. Similarly, Soutar et al. (9) also recommended RT alone as the treatment of choice for EMP of the H&N and proposed that radical surgery may not offer any additional benefit over RT alone in these patients. The role of adjuvant CT for patients with EMP also remains debatable. However, most series do not support the administration of CT for the majority of patients with EMP (3,10,17).
Demonstrating a dose-response relationship for LC in RT for EMP is difficult due to the rarity of the disease and the small number of in-field recurrences in some series (10,17,20). Based on a review of the literature, Mendenhall et al. (16) performed a dose-response analysis based on 81 patients with localized plasmacytomas and found that a threshold dose of 40 Gy was required for LC (69% for <40 Gy vs. 94% for ≥40 Gy, P=0.005). Some authors have proposed doses between 40 and 50 Gy for small lesions and higher doses for larger tumors (26,27). The data from Tsang et al. (10) demonstrated that larger EMP tumors (≥5 cm) were associated with a greater risk of local recurrence (treatment failed locally in 4/4 patients, including 2 patients who received 35 Gy, 1 who received 45 Gy, and 1 who received 50 Gy). These authors suggested that a dose of 35 Gy or less may not be sufficient for the LC of bulky tumors and that such tumors require higher doses and combined-modality treatment. In the guidelines recommended by the United Kingdom Myeloma Forum (UKMF) in 2004, the optimal radiation dose ranges from 40 to 50 Gy. EMPs <5 cm show an excellent chance for LC with doses of approximately 40 Gy in 20 fractions, whereas EMPs ≥5 cm show a higher risk of local failure and thus may require higher doses of approximately 50 Gy in 25 fractions (9).
In our series, the results revealed that the dose ≥45 Gy group exhibited superior 5-year LRFS, MMFS and PFS. Tournier-Rangeard et al. (12) reported on 17 patients with H&N EMP and similarly found that compared with doses <45 Gy, doses ≥45 Gy significantly improved the 5-year LC rate (100% vs. 55%, P=0.034) and the 5-year DFS rate (87.5% vs. 37.5%, P=0.056). Furthermore, the present study indicated that no local failure occurred among the patients who received postoperative RT at a dose ≥45 Gy; however, one patient who received postoperative RT with 39.4 Gy for macroscopic disease developed a local recurrence within the irradiated fields. The other one, who had a negative margin, had no local recurrence after postoperative RT at 40 Gy. The result indicated that postoperative RT of at least 40 Gy is preferable for macroscopic disease (28). Furthermore, Strojan et al. (7) also suggested that for patients receiving primary surgery, radiation doses of 40–50 Gy in 1.8–2.0 Gy daily fractions adjusted to the bulk of the tumor is sufficient for macroscopic disease and 36–40 Gy for microscopic disease.
The necessity of ENI for H&N EMP remains controversial. Some studies support a routine use of ENI due to a relatively high rate of regional nodal failure (up to 22%) or the excellent results of ENI (29,30). Other studies recommend ENI only in the case of bulky tumors or primaries localized in a rich lymphatic drainage area (2,12,14). However, many authors do not support a routine use of ENI based on the following reasons: a minimal risk of regional relapse (<4%), reduction of the risk for normal tissue damage, an opportunity for successful salvage RT following regional relapse, and the presence of nodal involvement which does not affect the survival outcomes (7,10,31). Strojan et al. (7) reported 14 H&N EMP patients with involved-site RT, and no regional failure occurred, even though 50% of the tumor originated from a rich lymphatic drainage area. They recommended limited-field RT for H&N EMP. Identical results were obtained by Skóra et al. (27), in which 14 H&N EMP patients received primary site and positive lymph nodes RT, 3 patients received ENI, and all remained regionally controlled. Similarly, in the present study, no regional failure occurred among the patients with H&N EMP in the RT+/–CT group, regardless of whether they received ENI, even though most of them (14/19) had tumors localized in the oral cavity, oropharynx and nasopharynx. Of note, involved-site RT will, in some patients, inadvertently cover a substantial part of the first-echelon cervical nodes adjoining the primary sites. Thus, combining the abovementioned research results with the guidelines (9,28), it is rational to apply involved-site RT to patients with H&N EMP.
Although this study contributes to the existing literature, corroborating many previously reported studies, we recognize several limitations of this study. First, this is a retrospective analysis of a relatively small number of patients, therefore, there may be inherent selection bias. Furthermore, there was a lack of uniformity in the tumor sites and treatments administered, which limits our ability to perform a rigorous statistical analysis of the pooled data. Thus, larger prospective clinical studies are expected to provide a higher level of evidence.
Conclusions
RT is the modality of choice for the management of patients with EMP. Involved-site RT of at least 45 Gy using conventional fractionation schedules should be considered. Patients with H&N EMP, tumor size <4 cm, age <50 years, and serum M protein negativity had better outcomes. Furthermore, progression to MM remains a challenging problem in this disease and was associated with poor OS, and local recurrence was associated with poor MMFS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121:749–57. [PubMed]
- Bachar G, Goldstein D, Brown D, et al. Solitary extramedullary plasmacytoma of the head and neck -- long-term outcome analysis of 68 cases. Head Neck 2008;30:1012–9. [PubMed] DOI:10.1002/hed.20821
- Finsinger P, Grammatico S, Chisini M, et al. Clinical features and prognostic factors in solitary plasmacytoma. Br J Haematol 2016;172:554–60. [PubMed] DOI:10.1111/bjh.13870
- Mendenhall WM, Mendenhall CM, Mendenhall NP. Solitary plasmacytoma of bone and soft tissues. Am J Otolaryngol 2003;24:395–9. [PubMed]
- Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer 1999;85:2305–14. [PubMed]
- Thumallapally N, Meshref A, Mousa M, et al. Solitary plasmacytoma: population-based analysis of survival trends and effect of various treatment modalities in the USA. BMC Cancer 2017;17:13. [PubMed] DOI:10.1186/s12885-016-3015-5
- Strojan P, Soba E, Lamovec J, et al. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol Biol Phys 2002;53:692–701. [PubMed]
- Sasaki R, Yasuda K, Abe E, et al. Multi-institutional analysis of solitary extramedullary plasmacytoma of the head and neck treated with curative radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:626–34. [PubMed] DOI:10.1016/j.ijrobp.2010.11.037
- Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol 2004;124:717–26. [PubMed]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys 2001;50:113–20. [PubMed]
- Dempewolf R, Lee JH. Extramedullary plasmacytoma presenting as a nasal mass in an immunosuppressed patient: treatment after failed primary radiotherapy. Ear Nose Throat J 2008;87:223–5. [PubMed]
- Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: A dose greater than 45 Gy to the target volume improves the local control. Int J Radiat Oncol Biol Phys 2006;64:1013–7. [PubMed] DOI:10.1016/j.ijrobp.2005.09.019
- Katodritou E, Terpos E, Symeonidis AS, et al. Clinical features, outcome, and prognostic factors for survival and evolution to multiple myeloma of solitary plasmacytomas: a report of the Greek myeloma study group in 97 patients. Am J Hematol 2014;89:803–8. [PubMed] DOI:10.1002/ajh.23745
- Creach KM, Foote RL, Neben-Wittich MA, et al. Radiotherapy for extramedullary plasmacytoma of the head and neck. Int J Radiat Oncol Biol Phys 2009;73:789–94. [PubMed] DOI:10.1016/j.ijrobp.2008.04.077
- Li QW, Niu SQ, Wang HY, et al. Radiotherapy alone is associated with improved outcomes over surgery in the management of solitary plasmacytoma. Asian Pac J Cancer Prev 2015;16:3741–5. [PubMed]
- Mendenhall CM, Thar TL, Million RR. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys 1980;6:1497–501. [PubMed]
- Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys 2006;64:210–7. [PubMed] DOI:10.1016/j.ijrobp.2005.06.039
- Gerry D, Lentsch EJ. Epidemiologic evidence of superior outcomes for extramedullary plasmacytoma of the head and neck. Otolaryngol Head Neck Surg 2013;148:974–81. [PubMed] DOI:10.1177/0194599813481334
- Kilciksiz S, Celik OK, Pak Y, et al. Clinical and prognostic features of plasmacytomas: a multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol 2008;83:702–7. [PubMed] DOI:10.1002/ajh.21211
- Dagan R, Morris CG, Kirwan J, et al. Solitary plasmacytoma. Am J Clin Oncol 2009;32:612–7. [PubMed] DOI:10.1097/COC.0b013e31819cca18
- Zhu Q, Zou X, You R, et al. Establishment of an innovative staging system for extramedullary plasmacytoma. BMC Cancer 2016;16:777. [PubMed] DOI:10.1186/s12885-016-2824-x
- Reed V, Shah J, Medeiros LJ, et al. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer 2011;117:4468–74. [PubMed] DOI:10.1002/cncr.26031
- Guo SQ, Zhang L, Wang YF, et al. Prognostic factors associated with solitary plasmacytoma. Onco Targets Ther 2013;6:1659–66. [PubMed] DOI:10.2147/OTT.S53248
- Kumar S. Solitary plasmacytoma: is radiation therapy sufficient?. Am J Hematol 2008;83:695–6. [PubMed] DOI:10.1002/ajh.21248
- Suh YG, Suh CO, Kim JS, et al. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol 2012;91:1785–93. [PubMed] DOI:10.1007/s00277-012-1510-6
- Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park) 2000;14:101–8, 111, discussion 111-2, 115. [PubMed]
- Skóra T, Pudełek K, Nowak-Sadzikowska J, et al. Effect of definitive radiotherapy on the long-term outcome in patients with solitary extramedullary plasmacytoma. Hematol Oncol 2017;35:317–22. [PubMed] DOI:10.1002/hon.2261
- Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11–31. [PubMed] DOI:10.1016/j.ijrobp.2015.01.009
- Mayr NA, Wen BC, Hussey DH, et al. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol 1990;17:293–303. [PubMed]
- Bolek TW, Marcus RB Jr, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys 1996;36:329–33. [PubMed]
- Michalaki VJ, Hall J, Henk JM, et al. Definitive radiotherapy for extramedullary plasmacytomas of the head and neck. Br J Radiol 2003;76:738–41. [PubMed] DOI:10.1259/bjr/54563070
