Efficacy of vinorelbine combined with low-dose methotrexate for treatment of inoperable desmoid tumor and prognostic factor analysis
Introduction
Desmoid tumor (DT) is also known as aggressive fibromatosis (AF) and desmoid type fibromatosis. It is a rare, fibroblast-origin, benign tumor, and accounts for 0.03% of all neoplasms, with the annual incidence of 2–4 cases per million (1). DT could occur all over the body. The most common areas are limbs and trunk (2). It tends to occur in female adults with a male/female ratio of approximately 1:3, and the average age and median age of patients were 31 years and 29 years respectively (1). DT could be stable, aggressive, or have spontaneous remission (3), and will not metastasize to lymph node and distant areas. But it is easy to recur locally due to its local invasive growth, rather than the satellite lesions and skip metastasis. Most local recurrences occur within the first two years after operation, but sometimes it may recur in the 12th year after operation (4).
The high recurrent rate of DT after resection makes it very difficult to treat. Peng et al. (5) reported 211 patients with DT and the 5-year recurrent rate is about 50% after surgery. Radiation therapy could improve local control of patients with positive margin, but its clinical application was restricted by the complications such as limb contracture, growth disorder, pathological fracture and sarcoma after radiation. Thus, radiotherapy is not suitable for young patients. Shin et al. (6) found that adjuvant radiotherapy may delay the recurrence of the tumor, although it seems to have no effect on the final relapse rate. For these patients who are not suitable for surgery and radiotherapy, systemic treatment is necessary. But the clinical data of systemic treatment of DT are still limited.
In the current study, we summarized the clinical manifestation, treatment effect and prognostic data of patients with inoperable and progressive DT, who were treated in the Department of Bone and Soft Tissue Tumors, Peking University Cancer Hospital & Institute.
Materials and methods
Patients
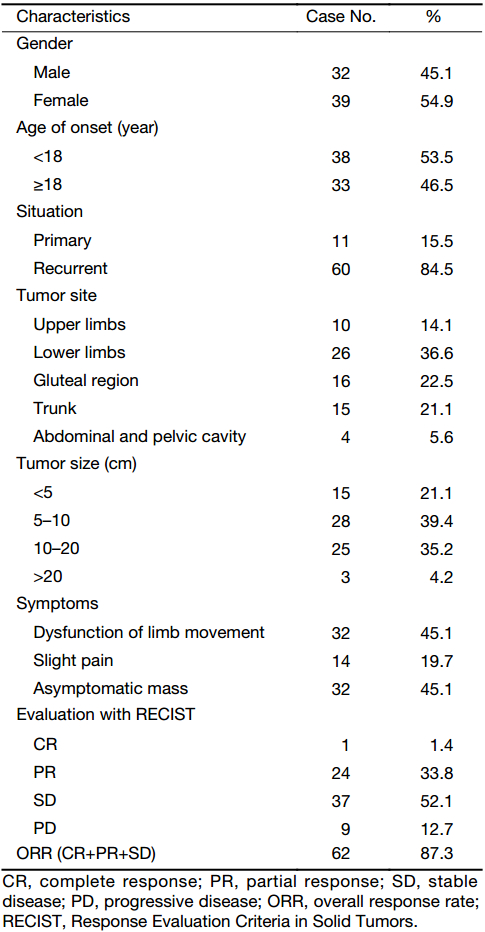
From November 2008 to April 2016, 71 patients with inoperable and progressive DT were treated in the Department of Bone and Soft Tissue Tumors, Peking University Cancer Hospital & Institute, and all the cases were pathologically diagnosed by experienced pathologists through core biopsy for primary cases and pathology consultation for recurrent cases. The clinical characteristics are described in Table 1. This study was approved by the Ethic Committee of Peking University Cancer Hospital and carried out in accordance with the approved guidelines.
Full table
Procedure and treatment
All the cases were evaluated by magnetic resonance imaging (MRI) or computed tomography (CT) scan before receiving chemotherapy to assess the possibility of radical resection and measure the tumor size by experienced radiologists. Patients with inoperable DT were enrolled in the study. The criteria for inoperable cases include: 1) the radical resection will cause massive defect of soft tissue and result in loss of limb function and huge change of appearance; 2) the radical resection will implicate the main nerves and vessels; 3) the tumor is invasive to the bone; 4) the patients refuse surgery; and 5) amputation is not considered. The exclusion criteria were: 1) the patients were with contraindications for chemotherapy; or 2) the patients refused chemotherapy.
Patients enrolled in the study received the chemotherapy of vinorelbine (NVB, 6–10 mg/m2, ivgtt, per week) combined with low-dose methotrexate (MTX, 30 mg/m2, po, per week; the total dose was divided into 5 pieces from the 1st day to the 5th day in a week). The planned whole chemotherapy period was 12 months.
The patients were followed up through outpatient and telephone by the same doctor (Li S), and the effect of chemotherapy was evaluated by MRI or CT scan to measure the tumor size. The assessment for response was taken before the chemotherapy, 6 and 12 months after initial chemotherapy and every 6 months after chemotherapy cessation. All patients were told to try to take all the reexaminations in Peking University Cancer Hospital. The tumor change was evaluated with Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) (7).
Statistical analysis
The tumor response to chemotherapy was observed according to RECIST guidelines. Kaplan-Meier method was used to calculate the relationship between survival data and the potential influencing factors, such as gender, age of onset, age of chemotherapy, tumor site and tumor size. Progression-free survival (PFS) analyses were performed by SPSS software (Version 16.0; SPSS Inc., Chicago, IL, USA) with a two-sided test at a 5% level of significance.
Results
The female/male ratio of the enrolled patients was about 1.2:1. Age of onset varied from 1 to 47 years, the median age was 14 years, and the ratio of adolescent and children to adult is about 1.2:1. Most patients (84.5%) suffered tumor recurrence. In 36 (50.7%) cases, tumors were in the limbs, and 45.1% suffered the dysfunction of limb movement, which is the main symptom for DT patients. The size of tumor (the maximum diameter) differed from 2 to 37 cm with a mean diameter of 9.3 cm. In 78.9% of cases, the tumor was more than 5 cm in diameter. The main cause was repeated resection and recurrence. The age when chemotherapy started varies from 3 to 52 years. The median age was 20 years, and 34 of 71 cases started chemotherapy when they were under 18 years. The median follow-up duration was 28 (range, 6–87) months.
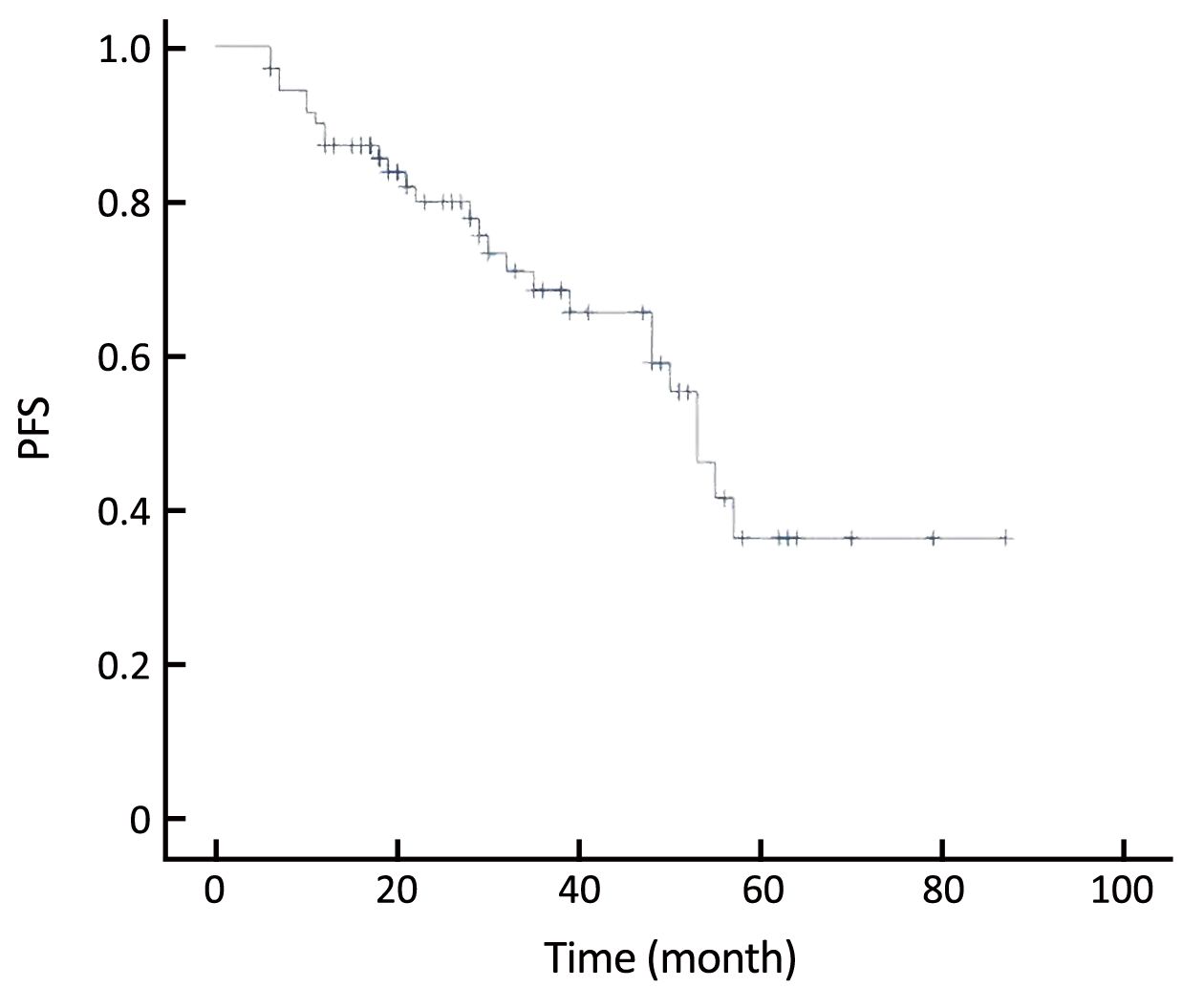
The chemotherapy duration of 14 cases was less than 1 year. The rest 57 patients completed at least 12 months of chemotherapy. When the chemotherapy finished, 1 case achieved complete response (CR), 24 achieved partial response (PR), 37 achieved stable disease (SD), and 9 had progressive disease (PD). The overall response rate (ORR) was 87.3% (Table 1). The PFS of the participants was from 6 to 87 months, and the 2-, 3- and 5-year PFS was 79.9%, 68.4% and 36.3%, respectively (Figure 1).
The common side effects included: nausea and vomiting, liver injury, bone marrow suppression and oral ulcers. Patients with elevation in hepatic transaminases (Grade 1 or more) were administered with reduced glutathione sodium (ivgtt) and bicyclol tablets (po). Patients with bone marrow suppression (Grade 2 or more) were administered with granulocyte stimulating factor. The antiemetics such as metoclopramide tablets were administered together with MTX. Patients with oral ulcers were treated with calcium folinate gargling and rinsing. The complication for most patients was tolerable.
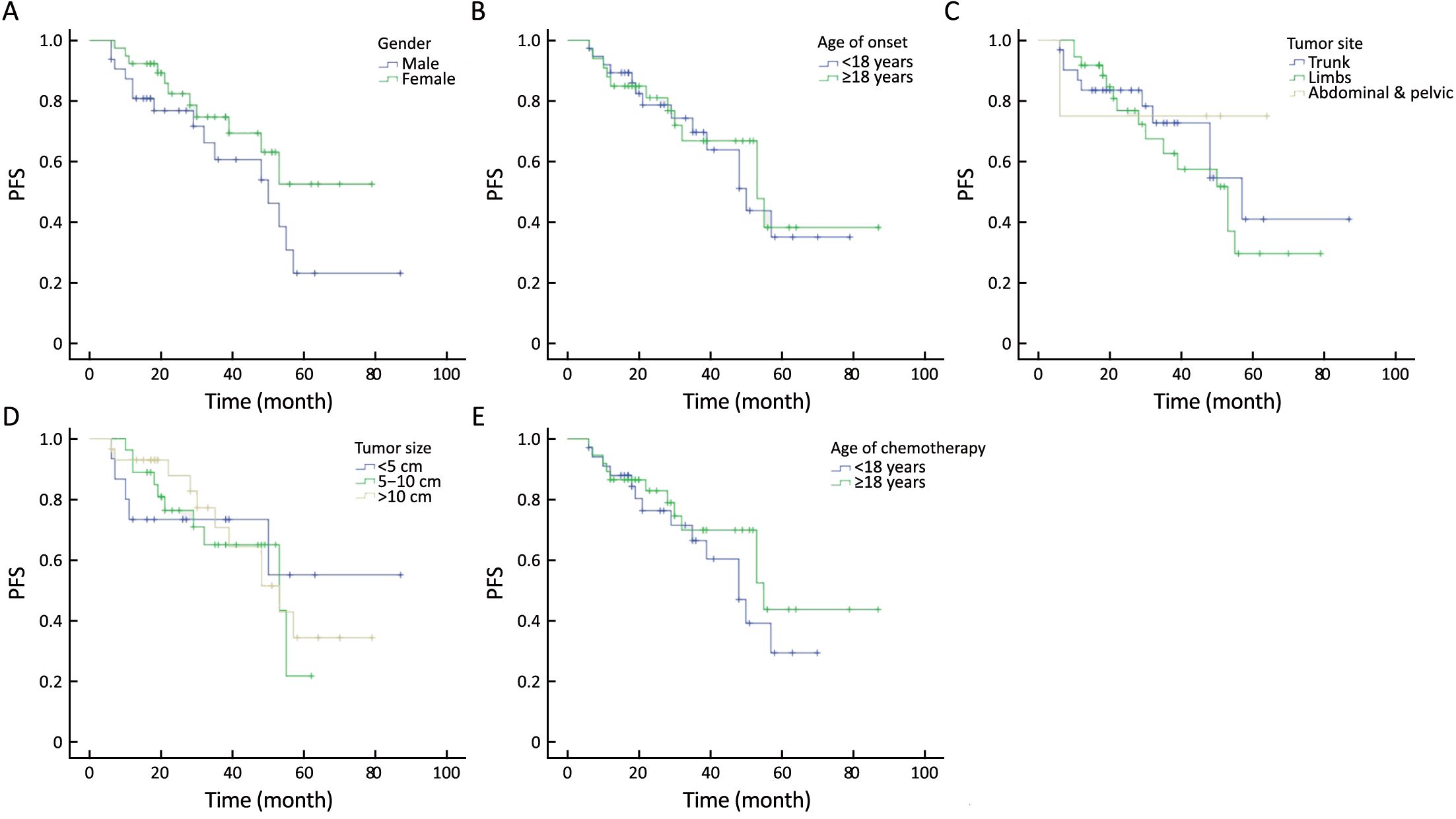
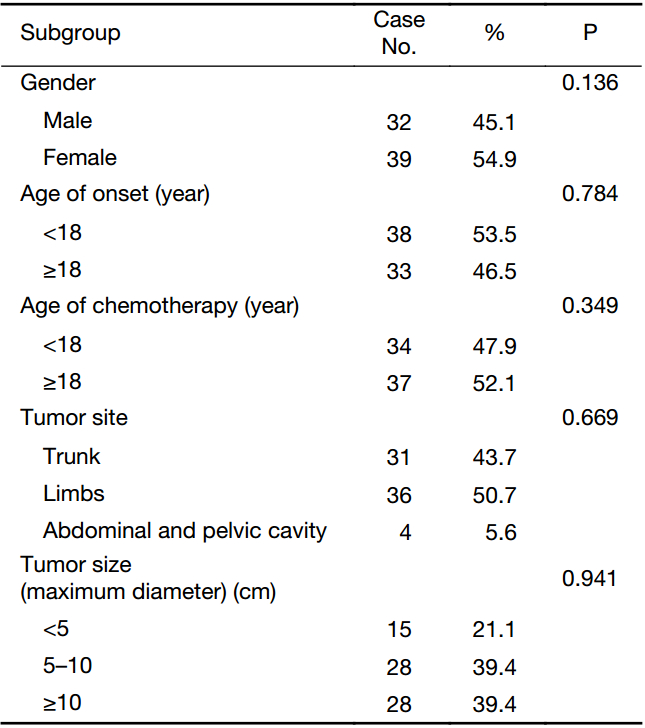
No significant difference was identified in PFS in subgroups of gender, age of onset, age of chemotherapy, tumor site and tumor size. PFS curves of different subgroups are showed in Figure 2 and the clinical characteristics of different subgroups are showed in the Table 2.
Full table
Discussion
Surgery
It is widely considered that surgery is the first choice for DT. Local control rate of patients with negative margin was better than those with positive margin (8). Zeng et al. (9) reported that tumor larger than 5 cm, extra-abdominal tumor and R1 resection status were associated with worse recurrence-free survival. The only factor associated with low recurrence is negative margin (10). But we cannot simply pursue negative margin under the risk of limb appearance and function impairment, because patients with positive margin still have chance for secondary radical surgery and radiotherapy. DT is not a lethal malignancy. The amputation is not a suitable choice for patients with unresectable DT. Some scholars pointed out that the indication of amputation including function-loss, severe pain, and invasion to main nerve, vessel and/or bone (11). In the current study, DT recurred in stump of some cases suffering amputation in other hospitals, and was well controlled after chemotherapy in authors’ hospital (Figure 3). Further, Gronchi et al. (2) investigated 203 patients of DT, and found the disease-free survival rate of primary patients is better than recurrent patients (10-year disease-free survival rate: 76% to 59%). Huang et al. (12) found admission status (primary or recurrent) had significant impacts on event-free survival. In this study, most patients with DT were recurrent cases when admitted in Peking University Cancer Hospital.
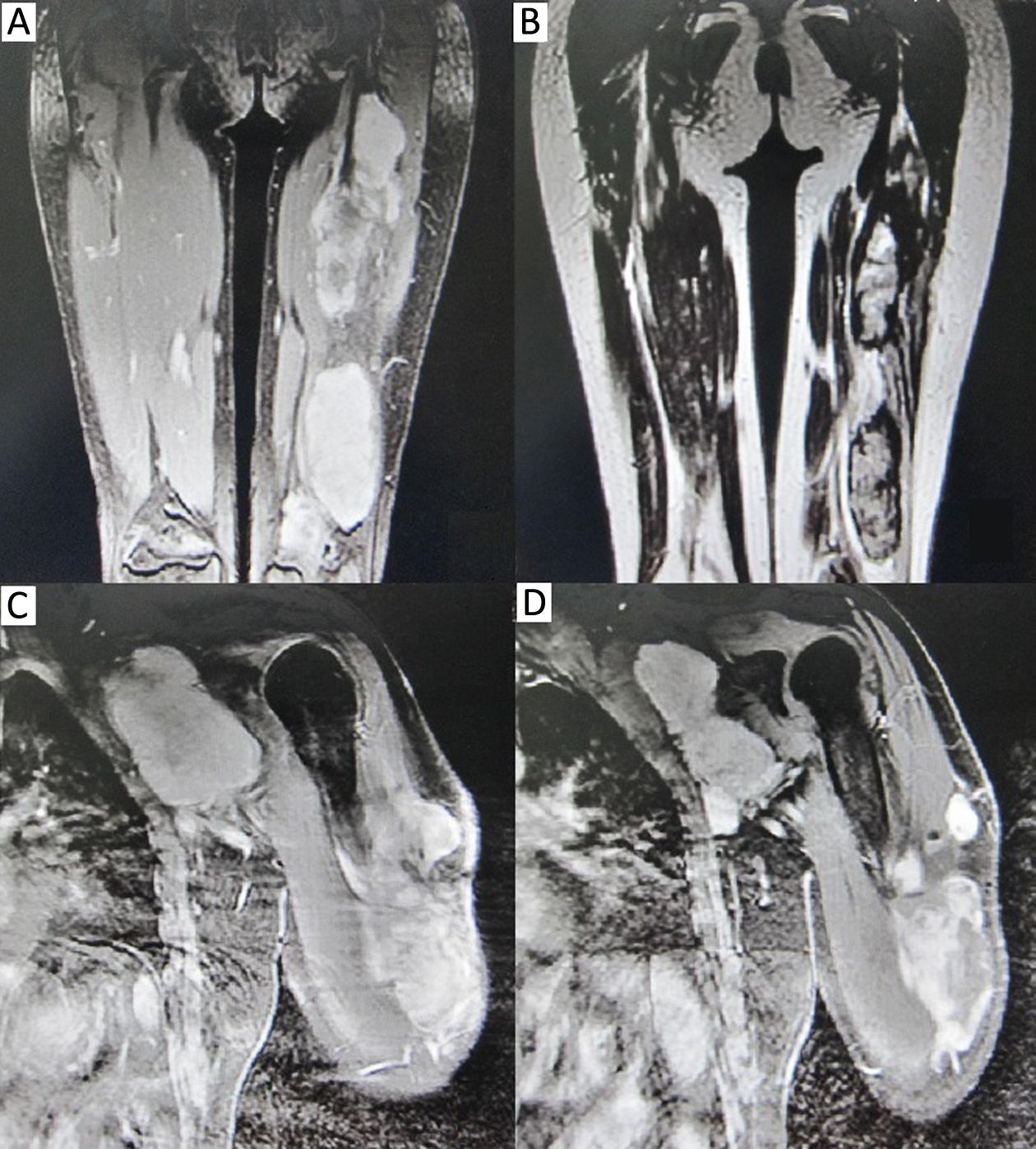
Recently, some researchers got the opposite conclusion about surgical margin after summarizing their clinical data. Woltsche et al. (13) and Soto-Miranda et al. (14) found the condition of the resection margin does not impact the local control rate of surgery, which indicated that DT has unique biological behavior, challenges the effectiveness of radical resection and highlights the need of adjuvant therapy like chemotherapy.
Radiotherapy
Radiotherapy is the second choice for DT. For patients with unresectable tumor and for whom the resection will cause severe functional impairment, radiotherapy could be considered. Kriz et al. (15) reported 37 patients received postoperative radiotherapy. The local control rate was 79%. However, many patients with DT were children and young people. For these young patients, radiotherapy may cause late and long-term complications, such as limb contracture, growth failure and secondary malignancy. Rutenberg et al. (16) reported 30 patients with DT (all <30 years old) treated with radiotherapy. Local-regional control rate in patients <18 years old at the time of radiotherapy was only 20%, while 63% in patients of 18–30 years old. Thus, for young patients with unresectable tumors, radical surgery and radiotherapy were inapplicable. The median age of onset of the patients in the current study was 14 years old, so radiotherapy was not selected for treating these patients.
Traditional pharmacotherapy
Besides resection and radiotherapy, pharmacotherapy is considered as third-line treatment of DT. Patients of DT who cannot receive radical surgery and radiotherapy should receive pharmacotherapy. Sulindac, non-steroidal anti-inflammatory drugs (NSAIDs), tamoxifen, toremifene, MTX and vinblastine (VBL), low-dose interferon, doxorubicin (DOX)-based regimens and imatinib were common used systemic therapy agents and recommended by National Comprehensive Cancer Network (NCCN) (17). Skapek et al. (18) reported 59 patients with measurable DT received tamoxifen and sulindac. Only 10 patients completed therapy. The estimated 2-year PFS was 36%. They thought such combination therapy showed relatively low activity as measured by response and PFS rates. In Hamada’s study (19), among 31 patients with extra-abdominal DT treated with meloxicam, 1 achieved CR, 7 achieved PR, 12 achieved SD, and 11 suffered PD. The authors found higher nuclear expression of β-catenin was significantly associated with poorer response. Penel et al. (20) reported 40 patients with unresectable and progressive symptomatic AF/DT treated with imatinib (400 mg/d for 1 year). The non-progression rates at 3, 6 and 12 months were 91%, 80% and 67%, respectively. The 2-year progression-free rate and overall survival rate were 55% and 95%. Nishida et al. (21) reported 15 patients with inoperable tumors who were resistant to meloxicam treatment were treated with MTX and VBL every other week. Six patients showed PR, and only 1 patient presented disease progression. Yoon et al. (22) reported 11 patients with AF treated with oral methotrexate (10 mg once per week). All of the patients had remission.
In Garbay’s study (23), combined chemotherapy was delivered in 44 (71%) cases, monotherapy in 18 (29%) patients, and 13 (21%) patients received an anthracycline-containing regimen. The most common nonanthracycline regimen was MTX-VBL combination (n=27). CR, PR, SD and PD were observed in 1 (1.6%), 12 (19.4%), 37 (59.6%) and 12 (19.4%) patients, respectively. Anthracycline-containing regimens appeared to be associated with higher response rate.
Yamamoto et al. (24) reported 3 patients with intra-abdominal DT who received low-dose dacarbazine (DTIC)-doxorubicin (DOX) (D-D) chemotherapy. Two of these patients achieved CR, and 1 patient achieved PR. Their study indicated that low-dose D-D chemotherapy was safe and effective for patients with intra-abdominal DTs. Ananth et al. (25) reported that in 5 children with progressive DF treated with liposomal doxorubicin, there was an average 4.5% reduction in tumor size.
New strategy of drug therapy
Jo et al. (26) evaluated the efficacy and safety of sunitinib in patients with advanced AF. Nineteen patients with pathologically proven AF were treated with sunitinib (37.5 mg/d) for 4 continuous weeks. Five patients achieved PR and 8 had SD. The 2-year PFS was 74.7% and OS was 94.4%. The main adverse events were neutropenia, diarrhea, and hand-foot syndrome. In 3 of 12 patients with mesenteric AF, mesenteric mass bleeding, bowel perforation and bowel fistula with tumor mass necrosis were observed. The author thought sunitinib showed potential anti-tumor activity and may be useful for the management of non-mesenteric AF.
Martin-Liberal et al. (27) first reported the outcome of two patients suffered progressive DT/AF and treated with pazopanib. Both patients achieved dramatic improvement in their symptoms and radiological signs of response. The clinical benefit lasted for more than 1 year. Then, Szucs et al. (28) reported 8 patients with DT treated with pazopanib, 3 of whom achieved PR and 5 achieved SD, and 6 patients derived clinical benefit from treatment in terms of improved function and/or pain reduction.
In a phase I study (29), 7 patients with DT were enrolled and treated with an oral gamma secretase inhibitor, PF-03084014, and 5 patients achieved PR.
Summary
Azzarelli et al. first reported 30 patients with DT who were treated with MTX and VBL. The response rate was 40%, and 10-year survival rate was 67% (30). However, it was found that peripheral neuropathy occurred in about 10% of patients treated with VBL. Neurotoxicity of NVB is less than that of VBL, and the therapeutic effect is the same (31). MTX+VBL therapy was used in 27 cases in Garbay’s study (23). The median chemotherapy duration lasted for 18 weeks, 4 patients achieved PR (15%), 14 achieved SD (52%), and 9 suffered PD (33%).
In our study, better outcomes were obtained (1 CR, 24 PR, 37 SD and 9 PD; ORR 87.3%), which might be the result of longer duration of chemotherapy (1 year vs. 18 weeks). Our chemotherapy protocol had less acute and long-term chemotherapy toxicity and was convenient for outpatients. There had been no common consensus on chemotherapy duration till now. But some scholars suggested that the chemotherapy should last for at least one year even without the radiological evidence of tumor response, unless tumor progressed and severe chemotherapy toxicity occurred (32). According to our experience and follow-up data, we thought that chemotherapy should last for at least 12 months and could be prolonged to 18 months regarding the status of patients (effects and side effects of chemotherapy).
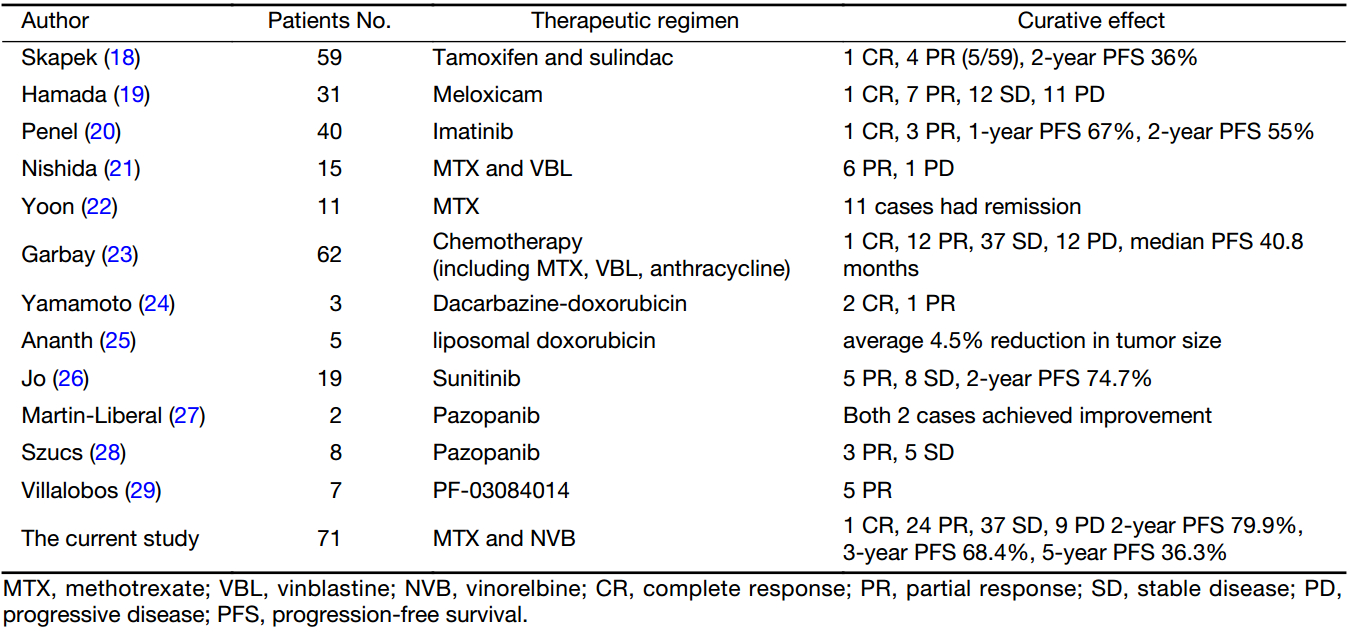
Compared to other studies (Table 3), the chemotherapy of MTX and NVB seemed to have better ORR and PFS for inoperable DT. But the limitation of the result is that the study is a single-arm retrospective study. Further controlled studies comparing this regimen with others are eagerly awaited, and multiple-center cooperation is recommended for conducting large-scale controlled studies.
Full table
Conclusions
For recurrent, unresectable and progressive DT, enough course of chemotherapy with vinorelbine combined with low-dose methotrexate was an optional choice for local control, especially for young patients who were not suitable for radiotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mendenhall WM, Zlotecki RA, Morris CG, et al. Aggressive fibromatosis. Am J Clin Oncol 2005;28:211–5. [PubMed]
- Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol 2003;21:1390–7. [PubMed] DOI:10.1200/JCO.2003.05.150
- Lazar AJ, Hajibashi S, Lev D. Desmoid tumor: from surgical extirpation to molecular dissection. Curr Opin Oncol 2009;21:352–9. [PubMed] DOI:10.1097/CCO.0b013e32832c9502
- Collins BJ, Fischer AC, Tufaro AP. Desmoid tumors of the head and neck: a review. Ann Plast Surg 2005;54:103–8. [PubMed]
- Peng PD, Hyder O, Mavros MN, et al. Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann Surg Oncol 2012;19:4036–42. [PubMed] DOI:10.1245/s10434-012-2634-6
- Shin SH, Ko KR, Cho SK, et al. Surgical outcome of desmoid tumors: adjuvant radiotherapy delayed the recurrence, but did not affect long-term outcomes. J Surg Oncol 2013;108:28–33. [PubMed] DOI:10.1002/jso.23343
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [PubMed] DOI:10.1016/j.ejca.2008.10.026
- Nuyttens JJ, Rust PF, Thomas CR Jr, et al. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer 2000;88:1517–23. [PubMed]
- Zeng WG, Zhou ZX, Liang JW, et al. Prognostic factors for desmoid tumor: a surgical series of 233 patients at a single institution. Tumour Biol 2014;35:7513–21. [PubMed] DOI:10.1007/s13277-014-2002-1
- Pignatti G, Barbanti-Bròdano G, Ferrari D, et al. Extraabdominal desmoid tumor: a study of 83 cases. Clin Orthop Relat Res 2000(375):207–13. [PubMed]
- Lewis JJ, Boland PJ, Leung DH, et al. The enigma of desmoid tumors. Ann Surg 1999;229:866-72; discussion 872-3.
- Huang K, Wang CM, Chen JG, et al. Prognostic factors influencing event-free survival and treatments in desmoid-type fibromatosis: analysis from a large institution. Am J Surg 2014;207:847–54. [PubMed] DOI:10.1016/j.amjsurg.2013.08.007
- Woltsche N, Gilg MM, Fraissler L, et al. Is wide resection obsolete for desmoid tumors in children and adolescents? Evaluation of histological margins, immunohistochemical markers, and review of literature. Pediatr Hematol Oncol 2015;32:60–9. [PubMed] DOI:10.3109/08880018.2014.956905
- Soto-Miranda MA, Sandoval JA, Rao B, et al. Surgical treatment of pediatric desmoid tumors. A 12-year, single-center experience. Ann Surg Oncol 2013;20:3384–90. [PubMed] DOI:10.1245/s10434-013-3090-7
- Kriz J, Eich HT, Haverkamp U, et al. Radiotherapy is effective for desmoid tumors (aggressive fibromatosis) - long-term results of a German multicenter study. Oncol Res Treat 2014;37:255–60. [PubMed] DOI:10.1159/000362398
- Rutenberg MS, Indelicato DJ, Knapik JA, et al. External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer 2011;57:435–42. [PubMed] DOI:10.1002/pbc.22916
- Soft tissue sarcoma. NCCN clinical practice guidelines in oncology. Available online: http://www.nccn.org
- Skapek SX, Anderson JR, Hill DA, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) Phase II Study. Pediatr Blood Cance 2013;60:1108–12. [PubMed] DOI:10.1002/pbc.24457
- Hamada S, Urakawa H, Kozawa E, et al. Nuclear expression of β-catenin predicts the efficacy of meloxicam treatment for patients with sporadic desmoid tumors. Tumour Biol 2014;35:4561–6. [PubMed] DOI:10.1007/s13277-013-1600-7
- Penel N, Le Cesne A, Bui BN, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol 2011;22:452–7. [PubMed] DOI:10.1093/annonc/mdq341
- Nishida Y, Tsukushi S, Urakawa H, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with desmoid tumors: relationship to CTNNB1 mutation status. Int J Clin Oncol 2015;20:1211–7. [PubMed] DOI:10.1007/s10147-015-0829-0
- Yoon GW, Kim JD, Chung SH. The analysis of treatment of aggressive fibromatosis using oral methotrexate chemotherapy. Clin Orthop Surg 2014;6:439–42. [PubMed] DOI:10.4055/cios.2014.6.4.439
- Garbay D, Le Cesne A, Penel N, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG). Ann Oncol 2012;23:182–6. [PubMed] DOI:10.1093/annonc/mdr051
- Yamamoto H, Oshiro R, Nishimura J, et al. Low-dose dacarbazine-doxorubicin therapy against intra-abdominal desmoid tumors. Oncol Rep 2013;29:1751–5. [PubMed] DOI:10.3892/or.2013.2345
- Ananth P, Werger A, Voss S, et al. Liposomal doxorubicin: Effective treatment for pediatric desmoid fibromatosis. Pediatr Blood Cancer 2017;64. [PubMed] DOI:10.1002/pbc.26375
- Jo JC, Hong YS, Kim KP, et al. A prospective multicenter phase II study of sunitinib in patients with advanced aggressive fibromatosis. Invest New Drugs 2014;32:369–76. [PubMed] DOI:10.1007/s10637-013-0059-0
- Martin-Liberal J, Benson C, McCarty H, et al. Pazopanib is an active treatment in desmoid tumour/aggressive fibromatosis. Clin Sarcoma Res 2013;3:13. [PubMed] DOI:10.1186/2045-3329-3-13
- Szucs Z, Messiou C, Wong HH, et al. Pazopanib, a promising option for the treatment of aggressive fibromatosis. Anticancer Drugs 2017;28:421–6. [PubMed] DOI:10.1097/CAD.0000000000000474
- Villalobos VM, Hall F, Jimeno A, et al. Long-term follow-up of desmoid fibromatosis treated with pf-03084014, an oral gamma secretase inhibitor. Ann Surg Oncol 2017. [Epub ahead of print].
- Azzarelli A, Gronchi A, Bertulli R, et al. Low dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer 2001;92:1259–64. [PubMed]
- Weiss AJ, Horowitz S, Lackmen RD. Therapy of desmoid tumors and fibromatosis using vinorelbine. Am J Clin Oncol 1999;22:193–5. [PubMed]
- Janinis J, Patriki M, Vini L, et al. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol 2003;14:181–90. [PubMed]