Outcome and prognostic factors in 110 consecutive patients with primary uterine leiomyosarcoma: A Rare Cancer Network study
Introduction
Uterine sarcomas are rare, and account for approximately 2%–6% of all malignant uterine tumors (1). Histologic classification of these neoplasms is based on the differentiation and/or growth pattern of the neoplastic cells and their presumed cell of origin. Primary uterine leiomyosarcoma (ULMS) is a rare type of cancer with a definite pathological identity amongst the different categories of uterine sarcoma. ULMS arises from the myometrial muscle, has a peak incidence occurring at the age of 50, and accounts for 30% of all uterine sarcoma (1). Looking at the prognostic factors, some investigators found the tumor size to be the most important prognostic factor, for patients with a tumor diameter larger than 5 cm presenting a poorer prognosis (2). However, a Gynecologic Oncology Group (GOG) study did not confirm these data, as in this study; only the mitotic index was the factor significantly related to progression-free interval (3). In a study by Oláh et al. (4), ULMS, matched for other known prognostic factors, presented a more aggressive behavior when compared to their carcinosarcoma counterparts. In most studies, the number of ULMS was low: in the study by the GOG, only 59 patients with ULMS were collected over a period of 9 years (3). Evans et al. (2) collected only 37 ULMS patients amongst all the patients who received a diagnosis of any type of uterine smooth muscle neoplasm diagnosed before 1977 at the University of Texas, M. D. Anderson Hospital. In the study by Oláh et al. (4), the data were obtained from the West Midlands Regional Cancer Registry, serving a catchment area of 2.6 million women. A total of 367 patients with a diagnosis of uterine sarcoma were identified, and included both ULMS and mixed mesodermal tumors (MMT). In a recent study by Davidson et al. (5), a total of 137 patients with ULMS were identified amongst a population of 294 patients diagnosed with uterine sarcoma in Norway from 1970 to 2000.
Due to its rare incidence, it is difficult to collect a sufficiently high number of patients to derive strong conclusions about the prognostic factors and the outcomes of patients affected by ULMS. The majority of recently published series dealing with uterine sarcoma not only report on small numbers of patients, but often do mix different subgroups such as carcinosarcomas, ULMS, and sarcomas arising in the endometrial stroma (5-9). One of the largest populations of ULMS patients from a single institution was published by Giuntoli et al. (10), in 2003; the authors have reported data on 208 patients collected at the Mayo Clinic over a period of 30 years.
The aim of the Rare Cancer Network (RCN, www.rarecancer.net), a multi-institutional international group, is to conduct retrospective studies in collecting data on rare forms of cancers (11). In this regard, the RCN launched a study aiming at identifying the outcomes and numerous potential prognostic factors in a population of patients affected by ULMS, most of them treated with surgery and adjuvant radiotherapy (RT). In this article, the results enrolling 110 patients from 19 RCN institutions are reported.
Materials and methods
Patient selection
One hundred and twenty-six consecutive patients treated between 1980 and 2000 in 19 member institutions of the RCN were collected in this retrospective study. All investigators obtained their own Institutional Review Board (IRB) approval for patient’s data collection.
Inclusion criteria consisted of a pathology report confirming the diagnosis of ULMS, patient aged 18–80 years, International Federation of Gynecology and Obstetrics (FIGO) staging assessment, complete information on treatment, and a minimum follow-up period of 6 months. All pathological reports and the staging assessments were centrally reviewed. Among the 126 cases received, 110 matched these criteria and were included in the analysis: there were 23 patients from France (2 centers), 20 from Italy (3 centers), 18 from Belgium (3 centers), 11 from the Netherlands (2 centers), 10 from Switzerland (4 centers), 8 from Spain (1 center), 7 from Israel (1 center), 7 from Poland (1 center), 3 from United Kingdom (1 center), and 3 from Turkey (1 center). The exclusion criteria for 16 women consisted of uterine sarcoma other than ULMS (n=4), or incomplete information on staging and treatment (n=12).
Staging procedures
Initial staging was performed by local and systemic investigations. Concerning local investigations, all patients received a full pelvic examination. Pelvic ultrasound was performed in 50 patients (45%), pelvic computed tomography (CT)-scan in 26 (24%), hysteroscopy in 8 (7%), laparotomy in 3 (3%), and pelvic magnetic resonance imaging (MRI) in 2 (2%). For the remaining patients, no further data were available about local investigation procedures. Systemic investigations were performed by abdominal ultrasound in 21 patients (19%), thoracic CT-scan and/or chest X-ray in 91 (83%), urography in 2 (2%), and rectoscopy in 2 (2%). For the remaining patients, no further data were available about systemic investigation procedures.
Follow-up
The median follow-up was calculated adopting the method described by Schemper et al. (12). Follow-up methods consisted of last clinical visit when available, or telephone call to either the patient or her general practitioner. Death certificates were obtained for deceased patients.
Statistical analysis
Means were compared by Student’s t-test, and 95% confidence intervals (95% CI) were calculated from standard errors. Proportions were compared using the Chi-square test for values greater than 5, and Fisher’s exact test for those less than or equal to 5. Kaplan-Meier product-limit estimates were used to evaluate the overall survival (OS), disease-free survival (DFS), local control (LC), locoregional control (LRC), and distant metastases-free survival (13). Time to any event was measured from the date of surgery. The events were all causes of death for OS, relapse or all causes of death for DFS, and local or locoregional relapse for LC and LRC, respectively. For distant metastases-free survival, the events included distant metastasis or all causes of death. Patients without any of the above-mentioned events were censored at their last follow-up. No patient was lost to follow-up (minimum follow-up period of 6 months). Information pertaining to the cause of death was always obtained from the clinical records and/or death certificates. No autopsies were carried out. Differences between groups were assessed using the log rank test. P<0.05 was considered statistically significant. Multivariate analysis was carried out using the Cox stepwise regression analysis to determine the independent contribution of each prognostic factor (14). Prognostic factors with a P-value less than 0.20 in univariate analysis were included in multivariate analysis. Table 1 summarizes the prognostic factors that have been studied in the univariate and multivariate analyses.

Full table
Results
Tables 2 and 3 summarize the data of the 110 patients enrolled in this analysis in terms of baseline characteristics (Table 2) and treatment (Table 3). Details on both full history and physical examination were available for 90 patients (82%). Twenty-three patients had no symptoms reported, and the diagnosis was made during a routine gynecological examination, while 87 patients presented at least one symptom. Median age was 54 (range, 19–77) years. Five patients presented a previous oncological history, including 3 breast cancer, 1 melanoma and 1 osteosarcoma, all of whom treated with curative intent. Fourteen women had a past medical history of benign uterine disease, twelve of them having undergone uterine surgery. Seventy-three women (66%) presented a previous history of pregnancy. In only 11 patients (10%), a previous hormonal treatment was reported. All patients underwent surgery as initial treatment. Seventy-five patients received adjuvant RT with different technologies (Linac, Co-60, Betatron, Neutron), 18 of whom had a combination of external-beam radiotherapy (EBRT) and brachytherapy (BRT). Five women underwent a focal treatment with palliative intent at the time of locoregional recurrence or distant metastasis. No data on the planned RT were available for the patients treated with a palliative intent.
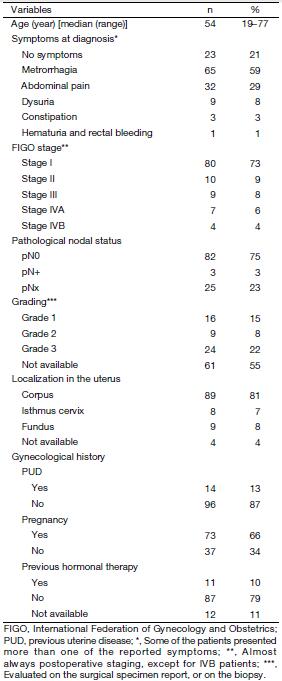
Full table
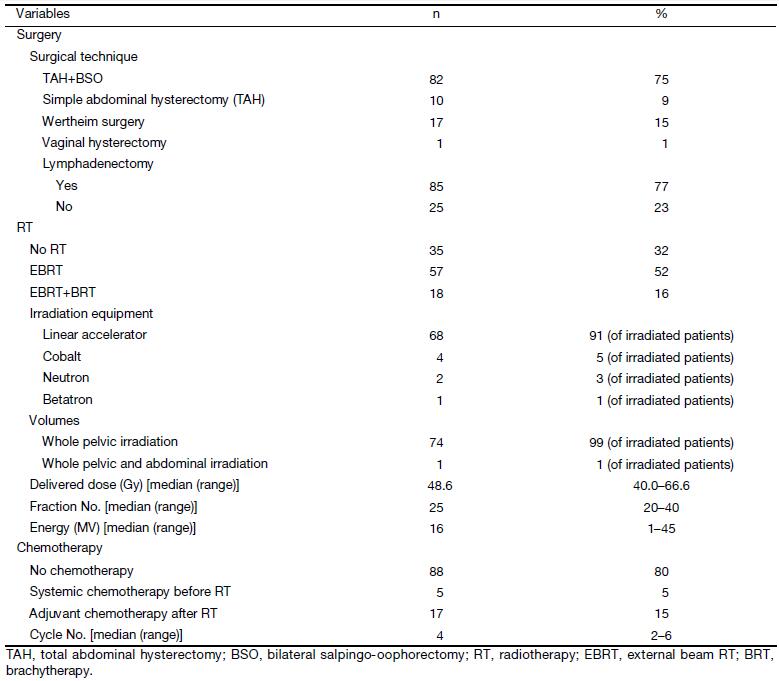
Full table
Systemic chemotherapy was delivered to 22 patients: 17 received it after RT with curative intent, whereas 5 patients who presented with a metastatic disease at diagnosis underwent chemotherapy before a palliative RT. The majority of patients received a combination of 2 or 3 drugs amongst the following: doxorubicine, epirubicine, ifosphamide, vincristine, or etoposide. One patient received two cycles of paclitaxel as a single drug.
Survival
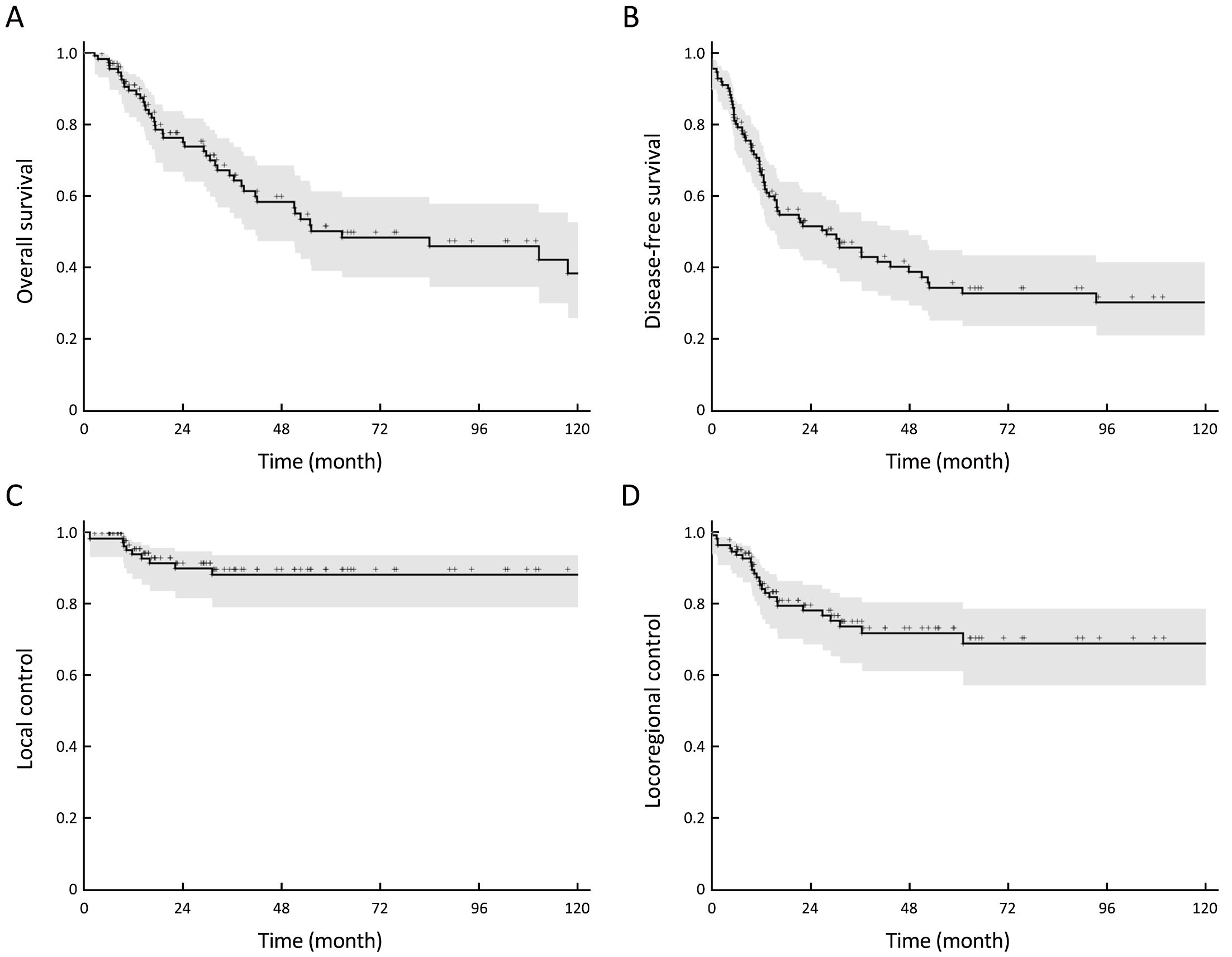
After a median follow-up period of 58 (range, 6–240) months, the 5-year OS (Figure 1A) and DFS (Figure 1B) were 50% (95% CI: 49%–61%) and 34% (95% CI: 24%–44%), respectively.
Patterns of failure
Local and locoregional failure
Four patients presented a local relapse, 16 patients a regional nodal relapse, and 6 patients a local and regional nodal failure. The 5-year LC (Figure 1C) and LRC (Figure 1D) were 88% (95% CI: 81%–95%) and 72% (95% CI: 62%–82%), respectively.
Systemic relapse
Altogether, 55 patients presented a systemic relapse. The 5-year distant metastases-free survival was 42% (95% CI: 31%–53%). The sites of failure were lung (n=43), liver (n=17), bone (n=15), brain (n=8), and the retroperitoneal lymph nodes (n=7). Of the 55 patients who had a systemic relapse, 9 (16%) had previously received adjuvant chemotherapy, whereas for the 55 patients without distant metastasis, 13 (24%) were given adjuvant chemotherapy. The risk of systemic relapse was not statistically different between the groups of locally relapsing and not relapsing patients (P=0.340).
Prognostic factors
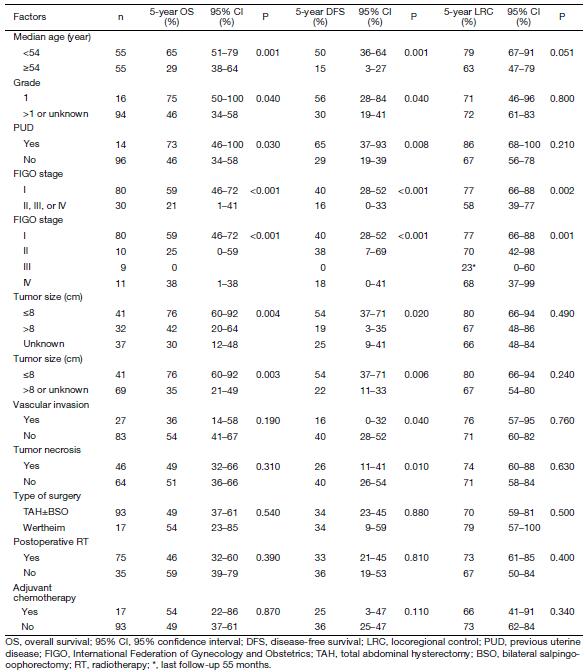
In univariate analysis (Table 4), age <54 years, previous uterine disease (PUD), grade 1 ( vs. others), FIGO stage (I vs. others, and FIGO I vs. II vs. III vs. IV), tumor size ≤8 cm (vs. others) were found to be favorable prognostic factors for OS and DFS (Figure 2); while vascular invasion and tumor necrosis were unfavorable prognostic factors only for DFS. FIGO stage I disease was the only favorable prognostic factor for LRC.
Full table
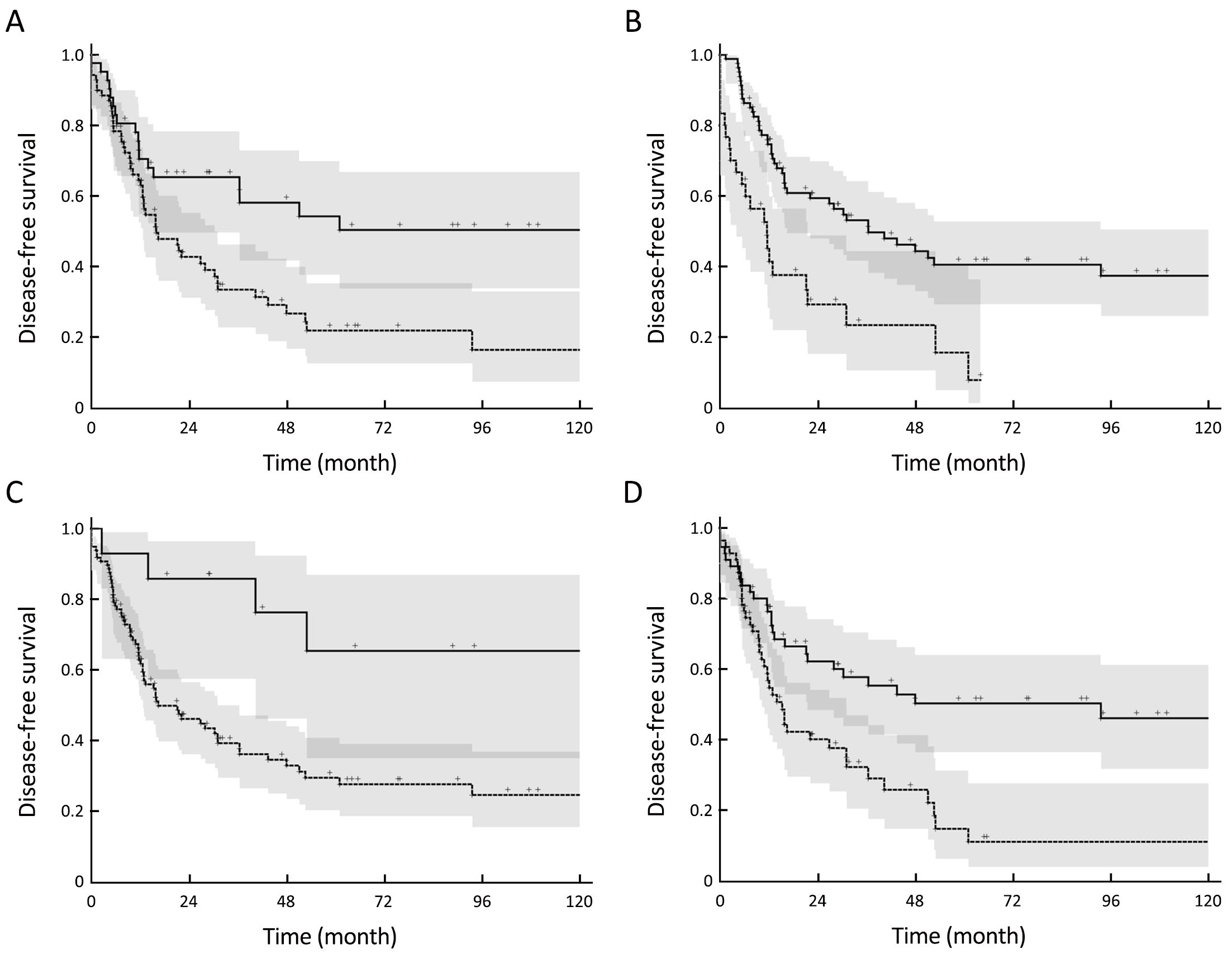
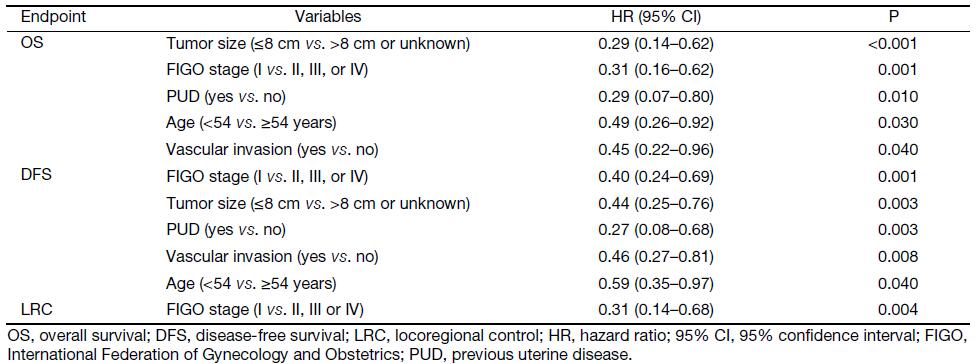
Table 5 shows the factors that independently and significantly influenced the considered endpoints in the multivariate analysis. Statistically significant independent favorable prognostic factors were younger age (<54 years), FIGO stage I, tumor size ≤8 cm, PUD, and no vascular invasion for OS and DFS. The FIGO stage was the only favorable independent factor influencing LRC. Adjuvant local or systemic treatments did not improve the outcomes. Noteworthy, none of the analyzed (univariate and multivariate analyses) variables influenced the LC. Interestingly, in univariate analysis, we found a better 5-year LC rate in patients having received also BRT compared to those having received only external pelvic irradiation (67%vs. 89%), but this difference was not statistically significant (P=0.060), and was not confirmed in the multivariate analysis. It should be noted that only 16% of patients received also BRT, which could probably explain these results.
Full table
RT and LC/LRC
The 5-year LC rate of irradiated patients was 86% (95% CI: 76%–96%) whereas it was 93% in non-irradiated patients (95% CI: 84%–100%) (P=0.400). The LRC was 73% (95% CI: 61%–85%) in irradiated patients and 67% in non-irradiated patients (95% CI: 50%–84%) (P=0.400) (Table 4). There was no impact of RT independently from the initial FIGO stage.
Surgical technique and LC/LRC
Surgical technique (Table 4) showed no significant impact on 5-year LC or LRC rates. The 5-year LC rate was 87% (95% CI: 78%–96%) with total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH+BSO; n=82) and 100% (95% CI: 98.7%–100%) with the Wertheim procedure (n=17; P=0.180). The 5-year LRC rate was 70% (95% CI: 59%–81%) with TAH+BSO and 79% (95% CI: 57%–100%) with the Wertheim procedure (P=0.500).
Radiation-induced early and late toxicity
Treatment was most often well tolerated. Using the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 scoring system (15), grade ≥3 toxicity was moderate, with 8 acute side effects (4 diarrhea, 2 hematological toxicity and 2 abdominal pain), and only 1 case of late complication (vaginal stenosis) was observed.
Discussion
We report one of the largest retrospective series specifically addressing the issue of numerous potential prognostic factors for ULMS. In our experience, the 5-year OS, DFS, LC, and LRC rates were 50%, 34%, 88%, and 72%; respectively. Because of the rarity of this cancer type, it is difficult to obtain large and/or prospective series: we needed the data from 19 European academic institutions to collect 110 cases, over a period of 20 years.
Most of the available studies present the same bias as in our analysis: because of the retrospective nature of the data, the conclusions that could be drawn are of limited value. However, in our series we have obtained complete data for 87% of the patients of the initial population of 126 patients, and for at least 90% of the data of the 110 patients enrolled in this analysis. In particular, treatment data were available for all patients. This makes the results of our analysis rather solid. Compared to other large published series, we decided to focus our analysis only on ULMS, and did not consider other histological subtypes, which are often mixed with ULMS in the other series.
The results in terms of clinical outcomes are comparable to those published by other institutions (16-21) including one very large series using the Surveillance, Epidemiology, and End Results Program (SEER) data (20). Surgery remains the standard initial approach to ULMS (1). The role of adjuvant treatments still remains controversial. A recent SEER-based retrospective analysis on 230 patients treated over a period of 17 years showed that the rate of use of RT and chemotherapy in the treatment of ULMS increased over the investigated period, but the authors could not show any significant survival advantage associated with either mode of adjuvant therapy (22). This study confirmed the available guidelines (1), which consider the role of adjuvant therapies to be controversial (23). Adjuvant RT seemed to be beneficial in some retrospective studies, but it had no impact on OS (10, 24, 27). Data from a retrospective analysis of 3,650 patients with uterine sarcoma (all histological subtypes) using the National Oncology Database showed that amongst the non-metastatic patients receiving definitive surgery (n=2,206) adjuvant RT was associated with an improved outcome compared with surgery alone [hazard ratio (HR)=0.4, P<0.001] (24). A multicenter analysis on 147 patients with ULMS showed a significant 5-year survival advantage for patients who received adjuvant RT (70% vs. 35%), but this survival advantage was not sustained as the curves crossed at 90-month follow-up. However, the pelvic recurrence rate was lower in the radiation group (18% vs. 49%; P=0.02) (25). Noteworthy, the median follow-up of 24 months limits the long-term value of the latter data. A large retrospective study on 208 patients treated at Mayo Clinic from 1976 through 1999 did not show any impact of RT on OS (10). A study by Livi et al. (27) on 141 patients (72 of them affected by ULMS) showed that postoperative RT with a total dose higher than 50 Gy significantly reduced the risk of local recurrence (P=0.001). Interestingly, a recent study on 69 ULMS patients published by Wong et al. (28) showed on multivariate analysis that RT independently reduced the risk of local relapse (HR: 0.28; 95% CI: 0.11–0.69, P=0.006) and increased OS (HR: 0.44; 95% CI: 0.23–0.85, P=0.014). One of the potential biases in analyzing the role of adjuvant RT is that most of the patients receiving RT presented a more aggressive disease at diagnosis, thus potentially lessening its impact. Nevertheless, the results of a randomized trial on 224 patients confirmed the data of the retrospective studies (29). This study enrolled patients affected by all uterine sarcoma subtypes, of which 103 patients were treated for a ULMS. All patients were operated on and then were randomized between either observation or pelvic RT (51 Gy in 28 fractions over 5 weeks). The initial analysis showed a reduction in local relapse (14 vs. 24, P=0.004), without any effect on either OS or PFS. Noteworthy, the positive impact of adjuvant RT was not confirmed in the subgroup of patients with ULMS (29).
Regarding adjuvant chemotherapy, there is little evidence in the literature supporting its use except for the carcinosarcoma histological subtype. Nevertheless, because of the high risk of systemic relapse, chemotherapy is usually delivered in the postoperative setting (1), despite the negative results of two phase III randomized trials (30,31). In our study, the risk of distant metastases was 55% at 5 years. Nine of the 55 relapsing patients received adjuvant chemotherapy, compared to 13 of the 55 non-relapsing patients. This difference was not statistically significant. Other series reported similar 5-year rates of distant relapses (16-18). Because of the high rate of systemic relapse, more effective systemic treatments represent a major issue in the therapeutic approach to ULMS. The results of our series confirmed that adjuvant RT and/or chemotherapy had no impact on the survival of patients.
In our study, a large number of potential prognostic factors were screened. Finally, on multivariate analysis, tumor size ≤8 cm, FIGO stage I, PUD, younger age (<54 years), and no vascular invasion confirmed their independent impact as significant favorable prognostic factors for OS and DFS. The positive impact of a less advanced FIGO stage on OS has been already shown in many retrospective and prospective studies (9,10,18,20,22,29,30).
Interestingly, PUD was associated with better OS and DFS on multivariate analysis: to the best of our knowledge, similar results have never been reported before. A potential explanation is that these patients underwent more frequent gynecological clinical controls and, therefore, could have benefited from an early diagnosis. Vascular invasion was associated with a worse prognosis, as reported by others (32-35).
In our series, a younger age (<54 years) was a positive prognostic factor. This result confirms the data of several studies available in the literature (4,17,20,36,37), even if some studies did not (27,38,39). One of the potential explanations could be the different hormonal status of the premenopausal women, as shown by a study by George et al. (40). However, more recent reports did not confirm the independent prognostic benefit of the menopausal status when patients’ age was taken into account (4). Thus, the reasons of the impact of age still need to be explained, even if, similar to the impact of the PUD reported in our study, it is possible that the more frequent gynecological controls of younger women led to the diagnosis of ULMS at an earlier stage.
Conclusions
This retrospective multicenter RCN study confirmed the poor prognosis of ULMS in spite of a good LC and LRC. An early diagnosis seemed to improve the prognosis of the patients, as an early FIGO stage had a positive impact on OS, DFS, and LRC. In our study, standard adjuvant local or systemic treatments, or more aggressive surgical procedures such as the Wertheim procedure, had no impact on the outcomes of the patients. The poor overall prognosis of this rare and aggressive disease indicates a strong need for newer and better combined approaches.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- PDQ® Adult Treatment Editorial Board. Uterine Sarcoma Treatment (PDQ®)-Health Professional Version. Bethesda: National Cancer Institute, 2015. Available online: http://www.cancer.gov/types/uterine/hp/uterine-sarcoma-treatment-pdq.
- Evans HL, Chawla SP, Simpson C, et al. Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer 1988;62:2239–47.
- Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 1993;71:1702–9.
- Oláh KS, Dunn JA, Gee H. Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: the result of a retrospective study of 423 cases of uterine sarcoma. Br J Obstet Gynaecol 1992;99:590–4.
- Davidson B, Kjæreng ML, Førsund M, et al. Progesterone receptor expression is an independent prognosticator in FIGO stage I uterine leiomyo-sarcoma. Am J Clin Pathol 2016;145:449–58. DOI:10.1093/ajcp/aqw030
- Parra-Herran C, Schoolmeester JK, Yuan L, et al. Myxoid leiomyosarcoma of the uterus: A clinico-pathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Pathol 2016;40:285–301. DOI:10.1097/PAS.0000000000000593
- Benson C, Ray-Coquard I, Sleijfer S, et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol Oncol 2016;142:89–94. DOI:10.1016/j.ygyno.2016.03.024
- Zhang J, Li T, Zhang J, et al. Clinical characteristics and prognosis of unexpected uterine sarcoma after hysterectomy for presumed myoma with and without transvaginal scalpel morcellation. Int J Gynecol Cancer 2016;26:456–63. DOI:10.1097/IGC.0000000000000638
- Roque DR, Taylor KN, Palisoul M, et al. Gemcitabine and docetaxel compared with observation, radiation, or other chemotherapy regimens as adjuvant treatment for stage I-to-IV uterine leiomyosarcoma. Int J Gynecol Cancer 2016;26:505–11. DOI:10.1097/IGC.0000000000000634
- Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management and adjuvant therapy. Gynecol Oncol 2003;89:460–9.
- Mirimanoff RO, Ozsahin M, Thariat J, et al. History of the rare cancer network and past research. Rare Tumors 2014;6:5462. DOI:10.4081/rt.2014.5462
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6.
- Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977;35:1–39.
- Cox DR. Regression models and life tables. J R Stat Soc Series B 1972;34:187–220.
- Common Terminology Criteria for Adverse Events v3.0 (CTCAE) available online at the address: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- Wang WL, Soslow R, Hensley M, et al. Histopathologic prognostic factors in stage I leiomyo-sarcoma of the uterus: a detailed analysis of 27 cases. Am J Surg Pathol 2011;35:522–9. DOI:10.1097/PAS.0b013e31820ca624
- Gadducci A, Landoni F, Sartori E, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol 1996;62:25–32. DOI:10.1006/gyno.1996.0185
- Echt G, Jepson J, Steel J, et al. Treatment of uterine sarcomas. Cancer 1990;66:35–9.
- Naaman Y, Shveiky D, Ben-Shachar I, et al. Uterine sarcoma: prognostic factors and treatment evaluation. Isr Med Assoc J 2011;13:76–9.
- Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyo-sarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008;112:820–30. DOI:10.1002/cncr.23245
- Kim WY, Chang SJ, Chang KH, et al. Uterine leiomyosarcoma: 14-year two-center experience of 31 cases. Cancer Res Treat 2009;41:24–8. DOI:10.4143/crt.2009.41.1.24
- Foley OW, Rauh-Hain JA, Clemmer J, et al. Trends in the treatment of uterine leiomyosarcoma in the Medicare population. Int J Gynecol Cancer 2015;25:453–8. DOI:10.1097/IGC.0000000000000372
- Ducie JA, Leitao MM Jr. The role of adjuvant therapy in uterine leiomyosarcoma. Expert Rev Anticancer Ther 2016;16:45–55. DOI:10.1586/14737140.2016.1115724
- Sampath S, Schultheiss TE, Ryu JK, et al. The role of adjuvant radiation in uterine sarcomas. Int J Radiat Oncol Biol Phys 2010;76:728–34. DOI:10.1016/j.ijrobp.2009.02.077
- Mahdavi A, Monk BJ, Ragazzo J, et al. Pelvic radiation improves local control after hysterectomy for uterine leiomyosarcoma: a 20-year experience. Int J Gynecol Cancer 2009;19:1080–4. DOI:10.1111/IGC.0b013e3181acae50
- Sahinler I, Atalar B, Tecer GM, et al. Postoperative radiotherapy in the treatment of uterine sarcomas: long-term results and analysis of prognostic factors. J BUON 2010;15:480–8.
- Livi L, Paiar F, Shah N, et al. Uterine sarcoma: twenty-seven years of experience. Int J Radiat Oncol Biol Phys 2003;57:1366–73.
- Wong P, Han K, Sykes J, et al. Postoperative radiotherapy improves local control and survival in patients with uterine leiomyosarcoma. Radiat Oncol 2013;8:128. DOI:10.1186/1748-717X-8-128
- Reed NS, Mangioni C, Malmström H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 2008;44:808–18. DOI:10.1016/j.ejca.2008.01.019
- Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol 2007;107:177–85. DOI:10.1016/j.ygyno.2007.07.070
- Omura GA, Blessing JA, Major F, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 1985;3:1240–5. DOI:10.1200/JCO.1985.3.9.1240
- Sait HK, Anfinan NM, EI Sayed ME, et al. Uterine sarcoma. Clinico-pathological characteristics and outcome. Saudi Med J 2014;35:1215–22.
- Nassar OA, Abdul Moaty SB, Khalil el-SA, et al. Outcome and prognostic factors of uterine sarcoma in 59 patients: single institutional results. J Egypt Natl Canc Inst 2010;22:113–22.
- Park JY, Kim DY, Suh DS, et al. Prognostic factors and treatment outcomes of patients with uterine sarcomas: analysis of 127 patients at single institution, 1989-2007. J Cancer Res Clin Oncol 2008;134:1277–87. DOI:10.1007/s00432-008-0422-2
- Pelmus M, Penault-Llorca F, Guillou L, et al. Prognostic factors in early-stage leiomyosarcoma of the uterus. Int J Gynecol Cancer 2009;19:385–90. DOI:10.1111/IGC.0b013e3181a1bfbc
- Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956–1992: incidence, survival and mortality. Eur J Cancer 1997;33:907–11.
- Mayerhofer K, Obermair A, Windbichler G, et al. Leiomyosarcoma of the uterus: a clinicopathologic multicenter study of 71 cases. Gynecol Oncol 1999;74:196–201. DOI:10.1006/gyno.1999.5436
- Silverberg SG. Leiomyosarcoma of the uterus. A clinicopathologic study. Obstet Gynecol 1971;38:613–28.
- Blom R, Guerrieri C, Stâl O, et al. Leiomyosarcoma of the uterus: A clinicopathologic, DNA flow cytometric, p53, and mdm-2 analysis of 49 cases. Gynecol Oncol 1998;68:54–61. DOI:10.1006/gyno.1997.4889
- George M, Pejovic MH, Kramar A. Uterine sarcomas: prognostic factors and treatment modalities — study on 209 patients. Gynecol Oncol 1986;24:58–67.
