Cycle number of neoadjuvant chemotherapy might influence survival of patients with T1-4N2-3M0 nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) originates from the epithelial cells covering the surface of nasopharynx (1), and it is a rare malignancy with an age-standardized incidence of <1/100,000 person-years, except in certain endemic regions such as South China ( 2,3). Due to anatomical complexity of NPC and its tendency to metastasize, radiotherapy instead of surgery is the main therapeutic modality (4,5). Since the advent of intensity-modulated radiation therapy (IMRT) and concurrent chemotherapy (CCT), the highest 5-year overall survival (OS) of NPC in the literature has now achieved 85.0% (6). Unfortunately, distant metastases remain the major causes of treatment failure. More than 25% of patients with advanced loco-regional disease eventually died of distant failure (7,8). A large scale meta-analysis in patients with head and neck squamous cell carcinomas showed that neoadjuvant chemotherapy (NACT) could significantly reduce distant metastases and improve survival (9). The roles of NACT in NPC remain controversial, though some clinical trials have recently indicated that patients treated with 2–3 cycles of NACT before concurrent chemo-radiotherapy (CCRT) have a better survival than those without NACT (10,11). It is known that the metastasis risk of NPC correlates with both the T and N stage, but the N stage is by far the most significant predicting factor (12,13). Even after multimodality treatment based on IMRT plus CCT, N2-3 NPC patients have a higher 5-year distant-metastasis rate (36.7% vs. 14.0%) and poor 5-year OS (66.0% vs. 84.3%) than N0-1 patients. And 51.4% of the distant metastases of N2-3 NPC occurred within one year (8). Thus these patients might obtain greater survival benefit from NACT through eradicating metastases. Additionally, circulating tumor cells are known to shed from the primary tumor and metastatic lymph nodes before, during or after treatment and form micrometastases, which are seeds for the subsequent growth of distant metastasis (14). We inferred that subclinical micrometastases were already present before treatment starting, in most cases with distant metastases shortly after removal of the primary and regional lesions. Hence, it was more appropriate to consider N2-3 NPC as a systemic disease instead of a local disease. The intensity of CCT which aims to enhance radiosensitivity of the local and regional lesions might not be sufficiently effective to control the pre-existing micrometastases. A more intensified systemic therapy such as NACT might be required. Furthermore, there are two issues of NACT to be resolved. First, almost all the previous studies on NACT of NPC enrolled stage III–IVB patients, including those with N0-1 diseases that might not need NACT. Second, there was no published study focusing on appropriate cycle number of NACT, especially for N2-3 patients. Therefore, we performed a prospective cohort study to evaluate the impact of various cycles of NACT on the survival of N2-3 NPC patients.
Materials and methods
Patient selection
Patients with pathologically diagnosed and previously untreated NPC in Sun Yat-sen University Cancer Center from January 1st 2008 to June 30th 2011 were initially considered. The ones who were younger than 70 years old and had N2-3 diseases would be consecutively included and prospectively observed. The stage of the patients was determined based on the Union for International Cancer Control/American Joint Cancer Committee TNM classification. For convenience of comparison, all the patients were restaged according to the 7th edition before analysis (15).
The exclusion criteria included: 1) Karnofsky performance score <80; 2) severe heart, lung, liver or kidney dysfunctions; 3) history of other malignancies; 4) prior chemotherapy or radiotherapy; 5) distant metastases before or during radiotherapy; or 6) application of adjuvant chemotherapy (ACT) or monoclonal antibody therapy.
All the patients were treated according to the guidelines of the National Comprehensive Cancer Network and Sun Yat-sen University Cancer Center for locally advanced NPC. The patients who were applied 4 cycles of NACT before their radical radiotherapy were referred to as the NACT=4 group. The NACT=0 group and the NACT=2 group were the patients treated without NACT, and the patients treated with NACT of 2 cycles before radiotherapy, respectively.
Through the frequency-matching technique, patients in the NACT=4 group were then matched in a ratio of 1:2:1 to those in the NACT=2 group and the NACT=0 group. Patients were considered to be matched when they had the same histological subtype (squamous cell carcinoma vs. non-keratinizing carcinoma vs. undifferentiated carcinoma), the same N stage (N2 vs. N3), the same NACT regimen (docetaxel plus cisplatin vs. cisplatin plus 5-fluorouracil) and the most approximate age. If there were several cases matched to the same patient, a random selection was made by the RANDBETWEEN function of the Microsoft Office Excel 2007 (Microsoft Co., Redmond, Washington, USA). Investigators were blinded to oncological outcomes during the selection process.
The entire patient enrollment procedure is summarized in Figure 1. All the patients in this study had detailed medical records including magnetic resonance imaging (MRI) of head and neck, whole-body bone scan and thoraco-abdominal computed tomography (or chest radiograph plus abdominal ultrasonography).
This study was approved by the institutional review board of Sun Yat-sen University Cancer Center. All patients signed informed consent before treatment.
Treatment strategy
In patients treated with NACT, NACT was administered every 3 weeks with the regimen comprised of docetaxel 75 mg/m2 d 1 plus cisplatin 75 mg/m2 d 1 or cisplatin 80 mg/m2 d 1 plus 5-fluorouracil 1,000 mg/m2 d 1–4. If any grade 3 to 4 (G3/4) blood, renal or hepatic disorders of Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0 were observed, NACT was delayed until the disorder recovered to grade 1 or disappeared, and the dose was decreased by 20% in the subsequent cycles. NACT was ceased at the appearance of intolerable adverse events, including a delay time of more than 2 weeks or twice appearance of any grade 4 adverse events.
Each of the patients in the NACT=4 group was planned to undergo a head and neck MRI to evaluate response of the metastatic cervical lymph nodes after 2 cycles of NACT. NACT would be continued if at least a partial remission was attained. If there was a stable or progression disease, NACT would be terminated. And then CCRT would be started instead. NACT would also be ceased after 3 cycles if lymph nodes became impalpable, a complete remission was achieved after the second cycle, or an intolerable adverse event appeared.
Regimen of CCT was single-agent cisplatin 40 mg/m2 weekly or 80 mg/m2 every 3 weeks throughout the whole procedure of radiotherapy.
All patients underwent radical radiotherapy. The target definition, delineation and dosage of radiotherapy were based on the standard of Sun Yat-sen University Cancer Center (See Appendix for details) (16,17).
Follow-up
Patients were followed up after treatment by outpatient interview or telephone. The intervals were 3 months for the first 3 years, 6 months for the 4th and 5th years, and 1 year thereafter. Follow-up was performed until death from NPC or December 31st 2016, whichever came first. Causes of deaths were confirmed by death certificates.
The primary endpoint of this study was OS, which was defined as the percentage of patients of a data set who survived after a defined period of time from pathologic diagnosis. The secondary endpoints included disease-free survival (DFS), local-recurrence-free survival (RFS) and distant-metastasis-free survival (MFS). These three endpoints were defined as the percentage of patients who had no corresponding events after a certain time period from diagnosis. The events for DFS included death, local recurrence and distant metastasis. And the events for RFS and MFS were local recurrence and distant metastasis, respectively.
Statistical analysis
Distribution balance of baseline clinical characteristics and acute toxicity among the NACT=4, NACT=2 and NACT=0 groups was assessed by the Chi-square test.
In survival analysis, sex (Male vs. Female), T stage (T3–4 vs. T1–2), CCT (Yes vs. No), IMRT (Yes vs. No) and NACT cycle (4, 2 vs. 0) were candidate variables. Each of these variables was first analyzed by a univariate analysis based on Kaplan-Meier approach to test whether it was a possible risk factor associated with survival. Differences in survival were assessed by a log-rank test. Then all the variables undergoing univariate analysis were also put into a multivariate analysis based on a Cox proportional hazards model as covariates. The hazard ratio (HR) and 95% confidence interval (95% CI) of each variable were calculated. The variables which maintained statistical significance were determined to be the independent prognostic factors. Finally, a multiple comparisons were made among the NACT=4, NACT=2 and NACT=0 groups.
The whole procedure of statistical analysis was done by IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA). A difference with two-sided P<0.05 was considered to be statistically significant.
Results
Baseline clinical characteristics
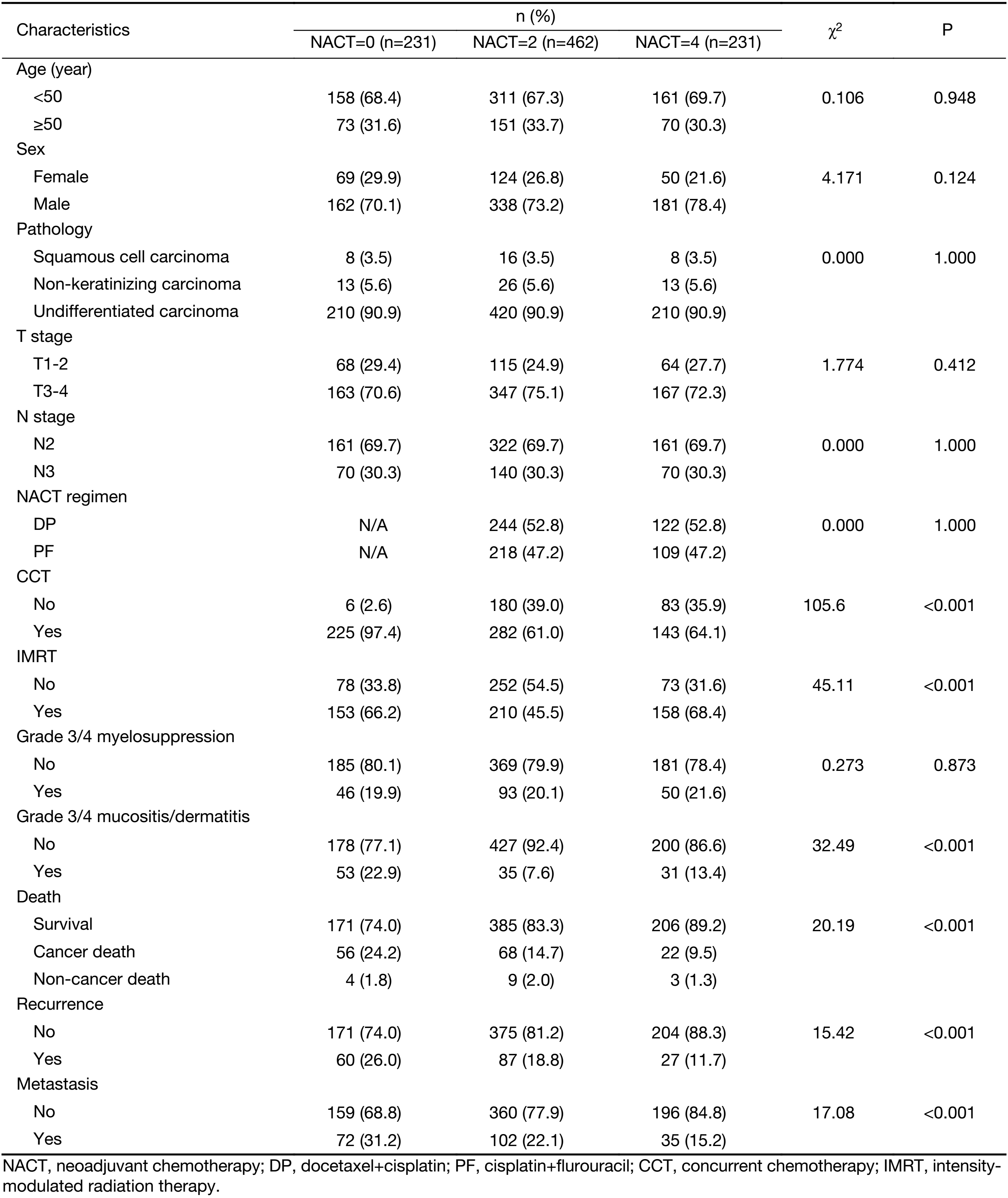
A total of 1,248 consecutive patients with N2-3 NPC were enrolled into this study. Of those, 84 patients were excluded for distant metastases before or during radiotherapy. Then a total of 1,164 patients with N2-3 non-metastatic NPC were eligible for analysis, including 249 cases in the NACT=4 group, 549 in the NACT=2 group and 366 in the NACT=0 group. After matching, there were 231, 462 and 231 cases in the NACT=4, NACT=2 and NACT=0 groups, respectively.
The distribution of the baseline clinical characteristics is shown in Table 1. Compared with the NACT=4 group, the NACT=0 group had more patients treated with CCT (97.4% vs. 64.1%, P<0.001), the NACT=2 group had similar proportion of patients treated with CCT (61.0% vs. 64.1%, P=0.438); the NACT=0 group had similar proportion of patients treated with IMRT (66.2% vs. 68.4%, P=0.620), the NACT=2 group had less patients treated with IMRT (45.5% vs. 68.4%, P<0.001). There was no difference in distribution of sex and T stage among the NACT=4, NACT=2 and NACT=0 groups.
Full table
Treatment results and acute toxicities
Up to December 31st 2016, a total of 162 patients died, in which 146 cases died of NPC. One hundred and seventy-four patients had local recurrence, and 209 patients had distant metastasis. The detailed treatment results are also shown in Table 1.
There was no grade 5 acute toxicity of CTCAE ver. 4.0 during treatment. The most common G3/4 toxicities were myelosuppression, mucositis and dermatitis. There was no significant difference among the NACT=4, NACT=2 and NACT=0 groups on number of patients with G3/4 myelosuppression (21.6% vs. 20.1% vs. 19.9%, P=0.873). Compared with the NACT=4 group, the NACT=0 group had more patients with G3/4 mucositis/dermatitis (22.9% vs. 13.4%, P=0.008), the NACT=2 group had less patients with G3/4 mucositis/dermatitis (7.6% vs. 13.4%, P=0.013).
Survival analysis
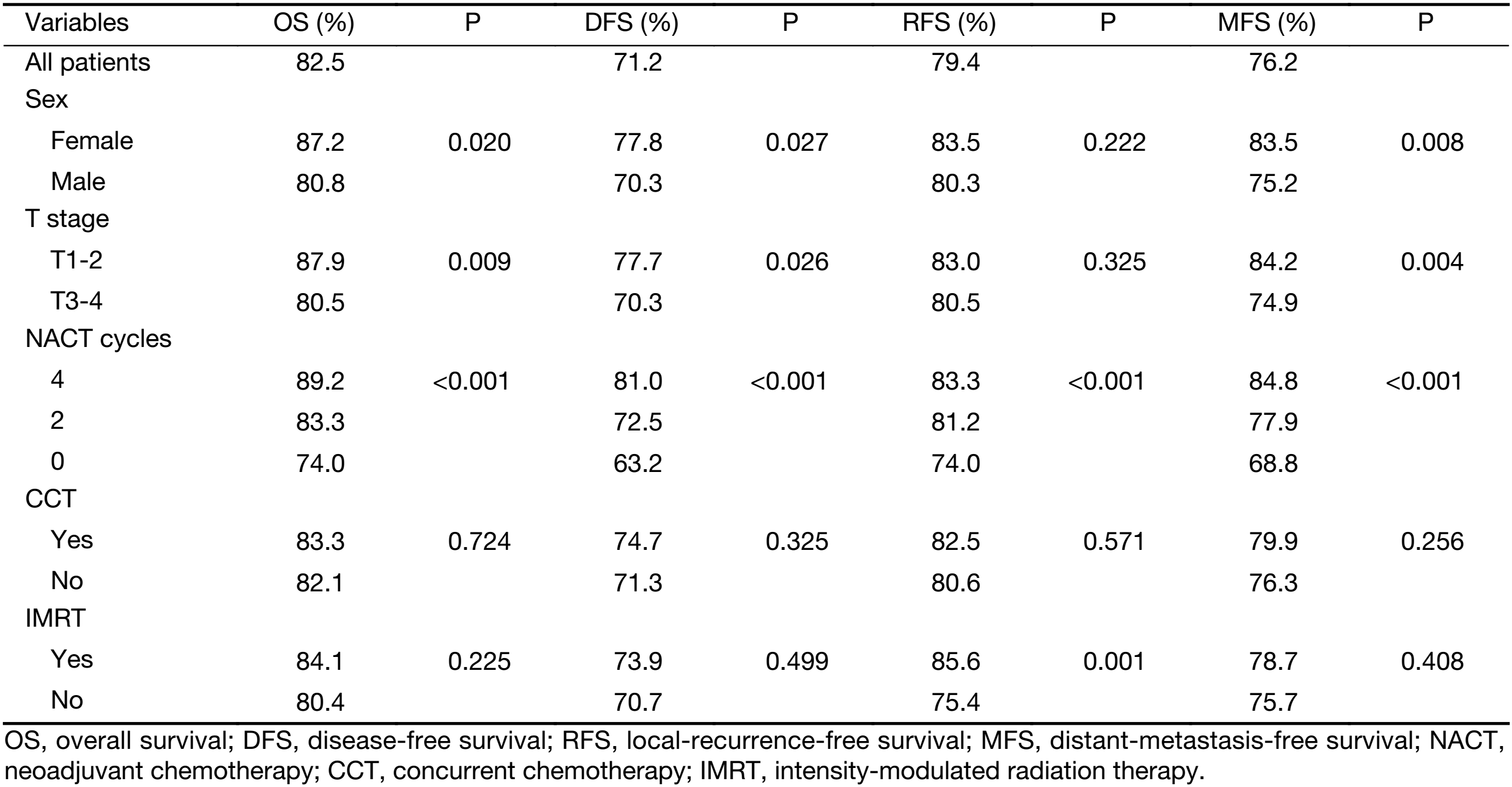
Results of univariate analysis are summarized in Table 2. Among the candidate variables we described above, the sex, T stage and NACT cycle presented as possible predictors of the OS (P=0.020, P=0.009 and P<0.001, respectively), the DFS (P=0.027, P=0.026 and P<0.001, respectively) and the MFS (P=0.008, P=0.004, P<0.001, respectively). The NACT cycle number, IMRT application appeared as possible predictors of the RFS (P<0.001 and P=0.001, respectively).
Full table
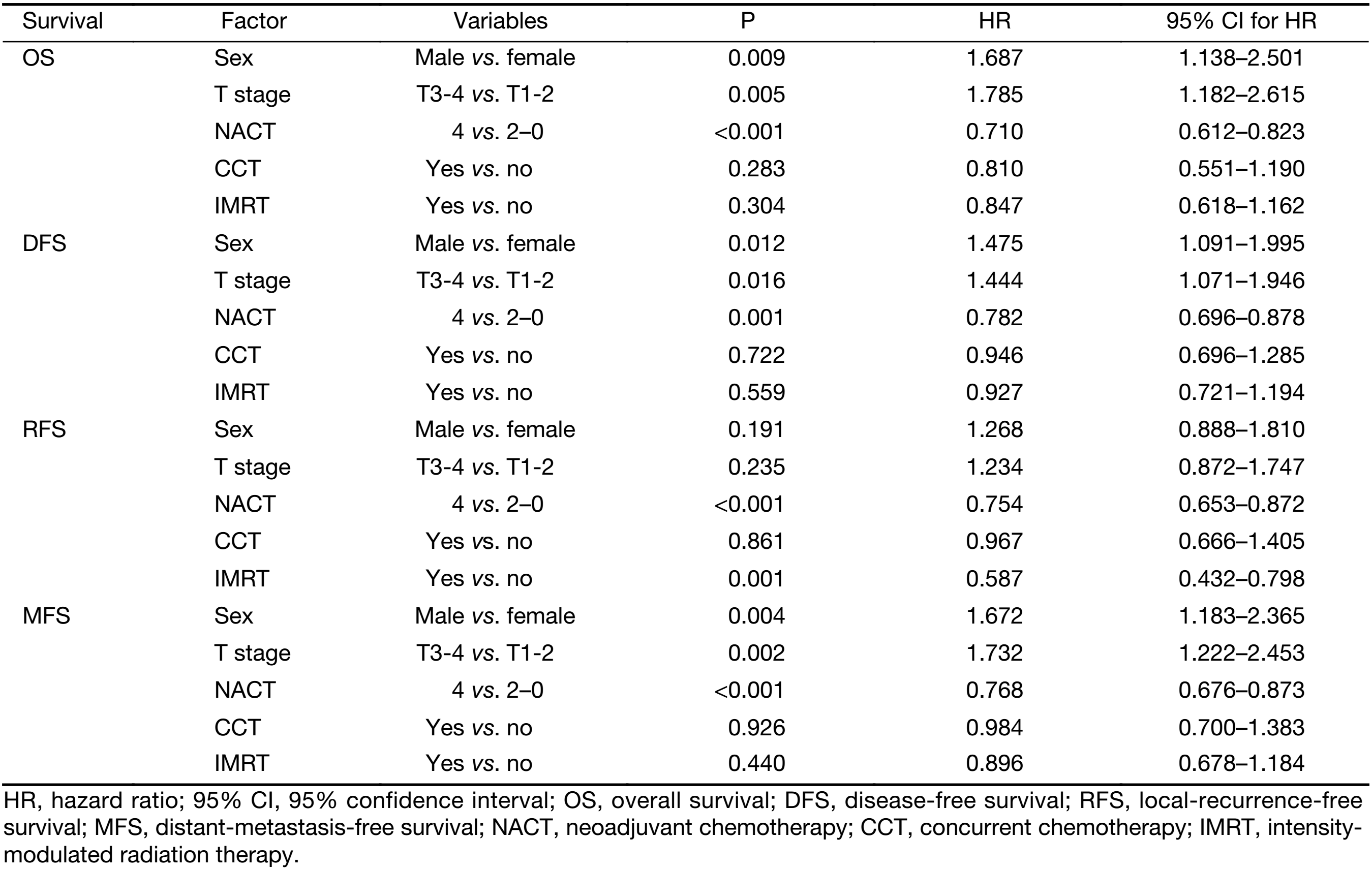
Table 3 shows the results of the multivariate analysis for the OS, DFS, RFS and MFS. The covariables included in this step were the same to the univariate analysis. The sex, T stage and NACT cycle number maintained statistical significance on OS (P=0.009, P=0.005 and P<0.001, respectively), DFS (P=0.012, P=0.016 and P=0.001, respectively), and MFS (P=0.004, P=0.002 and P<0.001, respectively). For the RFS, the NACT cycle number and IMRT application manifested statistical significance (P<0.001 and P=0.001, respectively). Thus, NACT cycle number (4 vs. 0–2) appeared to be an independent factor associated with improvement of the OS (HR, 0.710; 95% CI, 0.612–0.823), DFS (HR, 0.782; 95% CI, 0.696–0.878), RFS (HR, 0.754; 95% CI, 0.653–0.872) and MFS (HR, 0.768; 95% CI, 0.676–0.873).
Full table
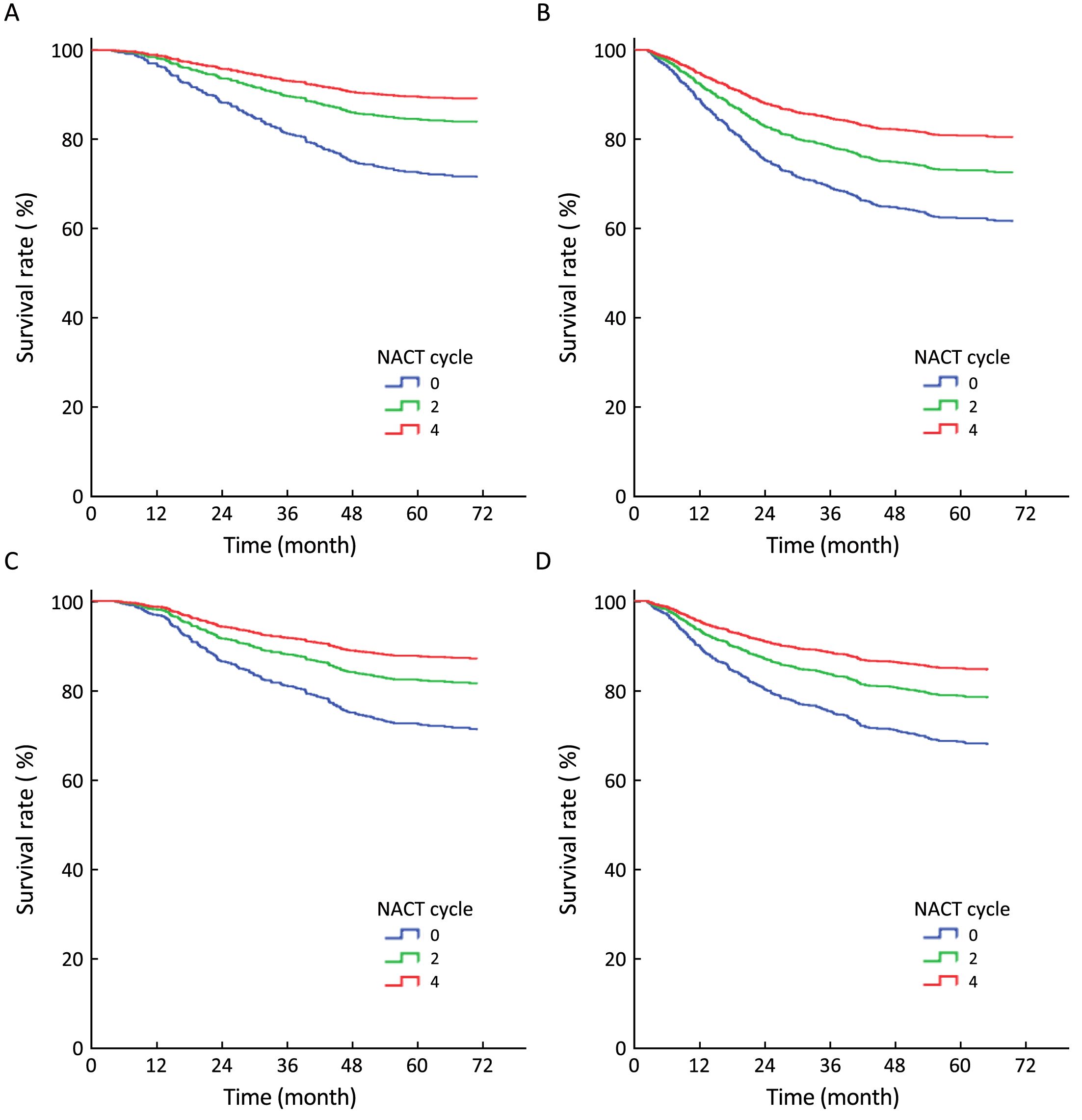
Multiple comparisons among the NACT=4, NACT=2 and NACT=0 groups were also made. The adjusted survival curves depicted through the COX model are shown in Figure 2. The OS, DFS, RFS and MFS of the NACT=4 group were all better than those of the NACT=2 group (P=0.013, P=0.007, P=0.021 and P=0.010, respectively) and the NACT=0 group (P<0.001, P<0.001, P=0.001 and P<0.001, respectively). Additionally, the OS, DFS, RFS and MFS of the NACT=2 group were all better than those of the NACT=0 group (P=0.004, P=0.005, P=0.004 and P=0.001, respectively).
Discussion
In order to further reduce the distant metastases of loco-regionally advanced NPC and improve survival, approaches have been made by oncological physicians on modifying sequence of chemotherapy from CCT to more intensified modes, including NACT plus CCT and CCT plus ACT. In a phase III multi-institutional randomized controlled trial, ACT after CCRT did not significantly improve the survival of patients with stage III–IVB NPC (18). Moreover, ACT plus CCT might bring more significant acute toxicities. A meta-analysis indicated that ACT plus CCT increased the incidence of G3/4 toxicities (19). Therefore, ACT was suggested currently to be performed with enough caution.
There are also many controversies on NACT in loco-regionally advanced NPC. First, its necessity is still equivocal. NACT was not an independent protecting factor of OS in many retrospective studies (20-22). Phase II trials of Tan et al. and Fountzilas et al. did not support the routine application of NACT either (23,24). However, in a phase II trial of Hui et al., 2 cycles of NACT before CCRT improved the 3-year OS (94.1% vs. 67.7%, P=0.012) in patients with stage III–IVB NPC (10). A benefit of 3-year OS brought by NACT was also seen in a recent phase III trial of Sun et al. (11). And a meta-analysis of Ouyang et al. revealed that NACT could effectively reduce distant metastasis rate and enhance OS (25). On the other hand, some studies were conducted to screen the suitable patients for NACT. Pretreatment serum level of Epstein-barr deoxyribonucleic acid (EBV-DNA) and the N stage were proven to be the most important risk factors for the distant metastasis of NPC (26). Patients with a high pretreatment EBV-DNA level or N2-3 diseases might be more eligible for NACT. A study conducted by Peng et al. revealed that NACT plus CCRT was a better treatment strategy for the patients who had high serum level of pretreatment EBV-DNA (27). Du et al. built a model to define high-risk patients who might benefit from NACT before CCT. High pretreatment EBV-DNA level and N2-3 diseases were two of the predicting factors included in the model (28). We achieved similar results in this study. For patients with N2-3 NPC, the cases treated with NACT of 4 cycles, and the cases treated with NACT of 2 cycles showed significantly improved OS, DFS, RFS and MFS than the cases treated without NACT. Especially, the difference of the MFS was more obvious (84.8% vs. 77.9% vs. 68.8%) than that of RFS (83.3% vs. 81.2% vs. 74.0%). Thus, N2-3 NPC, which is a marker of large tumor burden and high risk of distant metastasis, should be considered as one of the indications for NACT.
The suitable cumulative dose was another controversy of NACT in loco-regionally advanced NPC. Until now, the cycle number of NACT in most trials were 2–3 (11,12,23,24,29). There was no published study focusing on the appropriate cycle number of NACT for N2-3 NPC. Since N2-3 NPC has such a high risk of distant metastasis, it was hypothesized that a prolonged NACT which was more effective in eradicating subclinical metastases might also be more likely to improve survival of patients with these stages of disease. Actually, NACT of prolonged cycles (4–6 cycles) was proven to be effective for controlling distant metastasis and improving the clinical outcome of some solid tumors, such as breast and ovarian cancers (30,31). These tumors were thought to have high propensity to metastasize and considered as systemic diseases at diagnosis. Considering NPC itself, previous studies demonstrated that it was the cumulative dose of cisplatin in CCT but not the regimen that determined clinical outcome of NPC (32-34). After matching by well-known confounding prognostic factors, such as the N stage, we demonstrated in this study that the NACT cycle was an independent predicting factor for OS, DFS, RFS and MFS in patients with N2-3 non-metastatic NPC. Even though more patients treated without NACT received CCT, which was demonstrated to improve clinical outcome of loco-regionally advanced NPC (35). The OS, DFS, RFS and MFS of the patients treated with NACT of 4 cycles were all better than those of the patients treated with NACT of 2 cycles, and those of the ones treated without NACT. Furthermore, the survival rate increased as the cycle number of NACT increased. This result confirmed our hypothesis that N2-3 NPC was a systemic disease rather than a local disease, and NACT of prolonged cycles, such as 4 cycles, might be necessary.
Indeed, there were several limitations in this study. First, it was an observational study without random allocation of patients into groups treated with different cycles of NACT. Even having the same stage of disease, the patients undergoing completed NACT of 4 cycles had the potential possibilities of having better response with or without better tolerance to chemotherapy, compared with those who received NACT of 2 cycles or less. That might lead to some potential bias. Second, as we discussed above, EBV-DNA was one of the most important risk factors for the prediction of distant metastasis and should be taken into consideration when the association between NACT and survival of NPC was analyzed. The data of EBV-DNA were lack in patients enrolled before the year of 2009, mainly because it was not a routine lab examination in Sun Yat-sen University Cancer Center at that time. Third, the proportion of patients with CCT, and that of patients with IMRT was not balanced among the NACT=4, NACT=2 and NACT=0 groups. These two factors might be confounding factors in the process of survival analysis. The multivariate analysis involving the two factors could help to minimize this kind of biases effectively. Additionally, in this study, CCT and IMRT failed to appear as independent prognosticators for OS and MFS. To resolve the shortcoming and verify the results of this study, we have now been conducting a phase III randomized controlled trial (ClinicalTrials.gov Identifier: NCT02512315), including a uniform regimen of NACT, the planned testing of EBV-DNA, and the routine application of CCT and IMRT.
Conclusions
NACT before radical radiotherapy appeared to reduce distant metastasis and improve survival of non-metastatic N2-3 NPC patients. And its cycle number might be an independent factor associated with improved clinical outcome of these patients. This finding may be informative for clinicians to conduct clinical trials and direct treatment strategies, although further validation is needed.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (Grant No. 2017A020215157). The funding source had no role in the study design, data collection, analysis, interpretation or writing of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Appendix
Target definition, delineation and dosage standard of radiotherapy in Sun Yat-sen University Cancer Center
Conventional 2-dimensional radiotherapy consisted of two lateral opposing facio-cervical fields to cover the nasopharynx and the upper cervical lymphatic drainage region, and a lower anterior cervical field to cover the lower cervical region. After a dose of 36−40 Gy irradiated, two opposing lateral pre-auricular fields were used for the primary region, and anterior split neck fields were used for the cervical region instead. The primary tumor was given a total dose of 60−78 Gy, according to the tumor remission rate.
After a computed tomography (CT)-based simulation, the target volumes of intensity-modulated radiotherapy were delineated according to the guidelines of the International Commission on Radiation Units and Measurements (ICRU) Report 83. The gross tumor volumes (GTVs) included the primary tumor (GTVnx) and the enlarged lymph nodes (GTVnd) visible on magnetic resonance imaging (MRI). The clinical target volumes (CTVs) included the high-risk region (CTV1) and the intermediate-risk region (CTV2). The CTV1 encompassed the GTVnx with a radial margin of 0.5−1.0 cm (0.3−0.5 cm margin posteriorly), and covered the whole nasopharyngeal mucosa and a 0.5 cm submucosal region. The CTV2 encompassed the CTV1 plus a 0.5−1.0 cm margin (also 0.3−0.5 cm margin posteriorly) and the GTVnd, and contained the cervical lymphatic drainage regions. The planning target volumes (PTVs) for all GTVs and CTVs were constructed automatically by expanding the corresponding GTVs and CTVs by a 0.3−0.5 cm margin to overcome the immobilization and localization uncertainties. The irradiation was done in a conventional fractionation (1 fraction per day, 5 days per week). A total dose of 66−72 Gy was given to the PTV for GTVnx, 64−70 Gy to the PTV for GTVnd, 60−63 Gy to the PTV for CTV1, and 54−56 Gy to the PTV for CTV2.
References
- Li L, Gu M, You B, et al. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci 2016;107:1215–22. [PubMed] DOI:10.1111/cas.12989
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014;33:381–7. [PubMed] DOI:10.5732/cjc.014.10086
- Meng R, Wei K, Xia L, et al. Cancer incidence and mortality in Guangdong province, 2012. Chin J Cancer Res 2016;28:311–20. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.03.05
- Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 2015;33:3356–64. [PubMed] DOI:10.1200/JCO.2015.60.9347
- Li X, Chang H, Tao Y, et al. Revalidation of a prognostic score model based on complete blood count for nasopharyngeal carcinoma through a prospective study. Chin J Cancer Res 2016;28:467–77. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.05.01
- Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet 2016;87:1012–24. [PubMed] DOI:10.1016/S0140-6736(15)00055-0
- Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014;110:398–403. [PubMed] DOI:10.1016/j.radonc.2013.10.020
- Zang J, Li C, Zhao LN, et al. Prognostic model of death and distant metastasis for nasopharyngeal carcinoma patients receiving 3DCRT/IMRT in nonendemic area of China. Medicine (Baltimore) 2016;95:e3794. [PubMed] DOI:10.1097/MD.0000000000003794
- Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. [PubMed] DOI:10.1016/j.radonc.2009.04.014
- Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242–9. [PubMed] DOI:10.1200/JCO.2008.18.1545
- Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–20. [PubMed] DOI:10.1016/S1470-2045(16)30410-7
- Wu LR, Liu YT, Jiang N, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: An analysis of 614 patients from a single center. Oral Oncol 2017;69:26–32. [PubMed] DOI:10.1016/j.oraloncology.2017.03.015
- Liang ZG, Chen XQ, Niu ZJ, et al. Recommendations for updating T and N staging systems for nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. PLoS One 2016;11:e0168470. [PubMed] DOI:10.1371/journal.pone.0168470
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell 2006;127:679–95. [PubMed] DOI:10.1016/j.cell.2006.11.001
- Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edition. Berlin: Springer, 2010.
- Gao J, Tao YL, Li G, et al. Involvement of difference in decrease of hemoglobin level in poor prognosis of Stage I and II nasopharyngeal carcinoma: implication in outcome of radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1471–8. [PubMed] DOI:10.1016/j.ijrobp.2011.05.009
- Li JX, Huang SM, Jiang XH, et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiat Oncol 2014;9:87. [PubMed] DOI:10.1186/1748-717X-9-87
- Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163–71. [PubMed] DOI:10.1016/S1470-2045(11)70320-5
- Yan M, Kumachev A, Siu LL, et al. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis. Eur J Cancer 2015;51:1570–9. [PubMed] DOI:10.1016/j.ejca.2015.04.027
- Xia WX, Liang H, Lv X, et al. Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: A propensity score analysis. Oral Oncol 2017;67:167–74. [PubMed] DOI:10.1016/j.oraloncology.2017.02.026
- Song JH, Wu HG, Keam BS, et al. The role of neoadjuvant chemotherapy in the treatment of nasopharyngeal carcinoma: a multi-institutional retrospective study using propensity score matching analysis. Cancer Res Treat 2016;48:917–27. [PubMed] DOI:10.4143/crt.2015.265
- Zhang B, Hu Y, Xiong RH, et al. Matched analysis of induction chemotherapy plus chemoradiotherapy versus induction chemotherapy plus radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a multicenter study. Oncotarget 2017;8:14078–88. [PubMed] DOI:10.18632/oncotarget.13285
- Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952–60. [PubMed] DOI:10.1016/j.ijrobp.2015.01.002
- Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 2012;23:427–35. [PubMed] DOI:10.1093/annonc/mdr116
- Ouyang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol 2013;24:2136–46. [PubMed] DOI:10.1093/annonc/mdt146
- Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F] fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013;31:2861–9. [PubMed] DOI:10.1200/JCO.2012.46.0816
- Peng H, Chen L, Zhang Y, et al. Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status. Oncotarget 2016;7:24208–16. [PubMed] DOI:10.18632/oncotarget.8144
- Du XJ, Tang LL, Chen L, et al. Neoadjuvant chemotherapy in locally advanced nasopharyngeal carcinoma: Defining high-risk patients who may benefit before concurrent chemotherapy combined with intensity-modulated radiotherapy. Sci Rep 2015;5:16664. [PubMed] DOI:10.1038/srep16664
- Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 2015;121:1328–38. [PubMed] DOI:10.1002/cncr.29208
- Honkoop AH, Luykx-de Bakker SA, Hoekman K, et al. Prolonged neoadjuvant chemotherapy with GM-CSF in locally advanced breast cancer. Oncologist 1999;4:106-11. [PubMed]
- da Costa Miranda V, de Souza Fêde ÂB, Dos Anjos CH, et al. Neoadjuvant chemotherapy with six cycles of carboplatin and paclitaxel in advanced ovarian cancer patients unsuitable for primary surgery: Safety and effectiveness. Gynecol Oncol 2014;132:287–91. [PubMed] DOI:10.1016/j.ygyno.2013.12.002
- Lee JY, Sun JM, Oh DR, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG-HN10-02). Radiother Oncol 2016;118:244–50. [PubMed] DOI:10.1016/j.radonc.2015.11.030
- Peng H, Chen L, Li WF, et al. The cumulative cisplatin dose affects the long-term survival outcomes of patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy. Sci Rep 2016;6:24332. [PubMed] DOI:10.1038/srep24332
- Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol 2012;104:300–4. [PubMed] DOI:10.1016/j.radonc.2011.12.022
- Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656–66. [PubMed] DOI:10.1016/j.ejca.2010.10.026

