Prognostic factors of primary gastrointestinal stromal tumors: a cohort study based on high-volume centers
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and harbor functional mutations of KIT or platelet-derived growth factor receptor alpha (PDGFRA), which primarily drive tumor growth, progression and metastasis (1,2). They typically arise from the gastrointestinal tract but can also occur in the mesentery, omentum, pelvis, and retroperitoneum (3). Although surgical resection is still the cornerstone of management, patients with advanced tumors can now be successfully treated with molecular-targeted therapy (4-6). Indeed, GISTs have recently received considerable attention because of their sensitivity to the selective tyrosine kinase inhibitor (TKI) imatinib mesylate. Nevertheless, recurrence remains common and affects more than 50% of patients within 5 years of complete resection (7). Therefore, in this era of adjuvant TKI therapy, identifying the risk for recurrence has become increasingly important for clinicians.
Up to now, several risk-stratification systems have been proposed for operable GISTs that take into account the established independent risk factors of tumor size, mitosis count and tumor site (8,9). In addition, studies have revealed that the occurrence of tumor rupture either spontaneously or during surgery is associated with an increased risk for recurrence (10,11). Therefore, the recently proposed “modified National Institutes of Health (NIH) classification” that incorporates the occurrence of tumor rupture is commonly used in daily clinical practice (12). Furthermore, whether patients will benefit from adjuvant imatinib therapy remains controversial. Although several guidelines, such as the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) guidelines, suggest the use of adjuvant imatinib for intermediate- and high-risk patients, there are still no ideal methods to unequivocally identify which patients will benefit (7,13). It is therefore of vital importance to identify independent prognostic factors for accurate risk stratification that may help in determining the need for adjuvant imatinib and postoperative follow-up strategies. However, GIST is a distinct pathologic entity; the rarity of GISTs has limited its recognition and thus the identification of prognostic factors (14).
In the present study, we analyzed a large series of GISTs to gain a better understanding of their relative frequency, immunohistochemical expression and the clinicopathologic characteristics of patients with GISTs.
Materials and methods
Patient selection
A total of 2,570 consecutive GIST patients (January 2001–December 2015) were treated at the following four medical centers in China: Sun Yat-sen University Cancer Center, the Union Hospital Huazhong University of Science and Technology, Southern Medical University Nanfang Hospital and Guangdong General Hospital. All patient tumors were histologically classified as GISTs depending on their biopsy or their surgical specimen. The clinicopathologic and follow-up data (age, sex, medical history, tumor features and survival) were obtained from patient medical records.
The inclusion criteria were patients that had the following: 1) adequate paraffin-embedded tumor tissue sample for immunohistochemical analysis; 2) no other synchronous malignancy or multiple GISTs; 3) no preoperative imatinib or chemoradiotherapy; and 4) a complete set of clinicopathological and follow-up data. Furthermore, we excluded individuals for whom age, sex or general tumor features were unknown, or patients who died within one month after surgery.
Immunohistochemistry
The expression levels of c-Kit, CD34, delay of germination 1 (DOG-1), S-100, smooth muscle actin (SMA) and desmin were tested using streptavidin-peroxidase immunohistochemical staining. The immunohistochemical staining was performed on the representative 5-μm-thick tissue sections that were cut from formalin-fixed paraffin blocks. These tissue sections were pretreated at room temperature according to the requirements of the primary antibodies. Staining was carried out on an automated staining instrument (Ventana Medical Systems, Tucson, AZ, USA), according to the instruction manual. Diaminobenzidine was employed as the chromogen to identify the immunoreactivities in the present study.
Follow-up procedures
The patient follow-up information was obtained from hospital records. Of 2,168 resected patients, 1,441 had complete follow-up data. The follow-up data were routinely gathered annually for very low- or low-risk patients and every 6 months for intermediate- or high-risk patients. Postoperatively, patients were followed up with routine chest X-ray, endoscopy, and dynamic abdominal-pelvic computed tomography scan. Recurrence or metastasis was diagnosed according to clinical, radiologic, or endoscopic findings of disease. The final follow-up date for the study was February 2016. In the present study, we used overall survival (OS) as the primary outcome. OS was calculated from the date of surgery to the date of either death or last available follow-up.
Ethics statement
This study complied with the standards of the Declaration of Helsinki and was approved by the Ethical Committee of Sun Yat-sen University Cancer Center. No informed consent (written or verbal) was obtained for the use of the retrospective tissue samples from patients, some of whom were deceased. Informed consent was deemed unnecessary by the Ethical Committee, and all samples and information were anonymous.
Statistical analysis
The data were processed with IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA). The results were presented as the means and 95% confidence intervals (95% CI). The OS rate was estimated using the Kaplan-Meier method, and significant differences among groups were determined using a log-rank test. Variables considered significant at the 0.1 level in the univariate or unadjusted analysis were included in a final multivariate Cox proportional hazards model. All variables were assessed for interaction and co-linearity. Two-sided tests of statistical hypotheses that produced a P value <0.05 were considered statistically significant. All data in our study have been recorded at Sun Yat-sen University Cancer Center for future reference (No. RDDA2017000341).
Results
Demographics
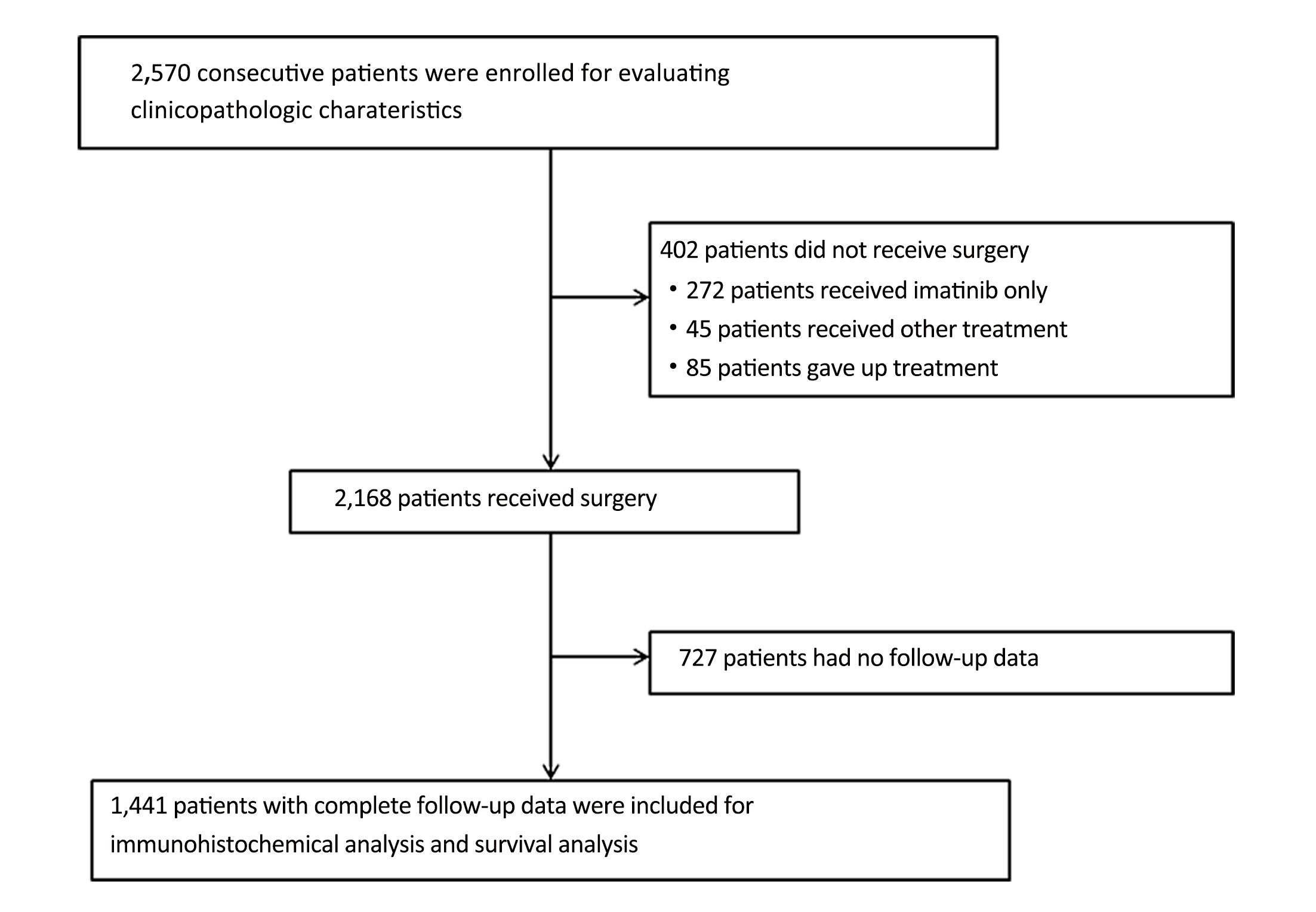
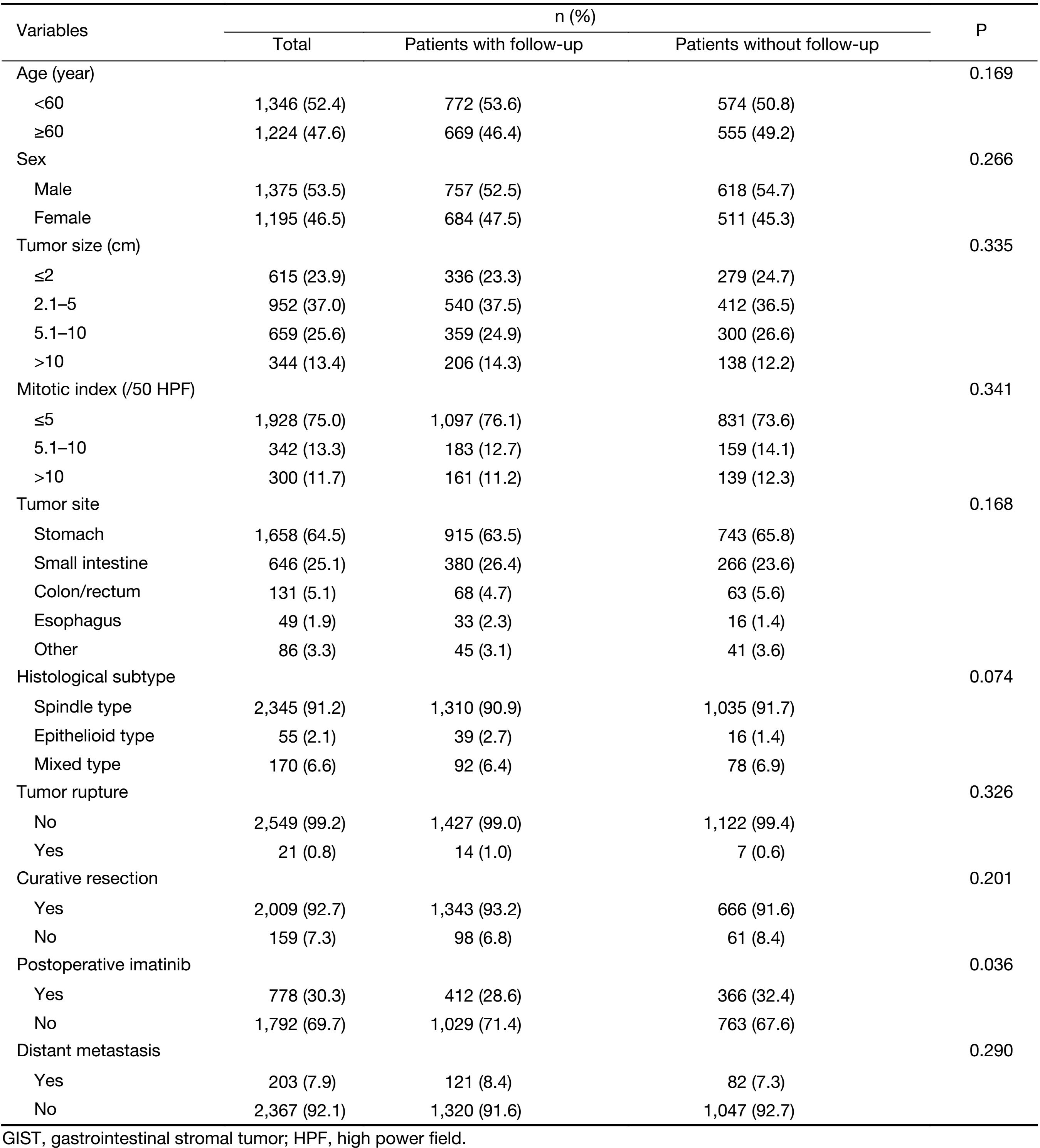
As shown in Table 1, 2,570 patients with GISTs were enrolled in the study. Of the patients, 1,375 (53.5%) were male, and 1,195 (46.5%) were female, with a ratio of 1.15:1. The median age of the patients was 58 (range, 18–95) years at the time of GIST detection. Only 215 patients (8.4%) were younger than 40 years. Primary sites included the stomach (64.5%), small intestine (25.1%), colorectum (5.1%) and esophagus (1.9%), as well as extra-gastrointestinal sites (3.3%), including the mesentery, omentum, pelvis, and retroperitoneum. Of 2,168 patients who underwent resection, 2,009 (92.7%) received curative resection, and 159 (7.3%) received palliative resection. Furthermore, 1,441 (56.1%) patients had complete follow-up data and 1,129 (43.9%) patients had no follow-up data. There were no statistically significant differences between the two groups with respect to the clinicopathologic characteristics (All P>0.05;Table 1) other than postoperative imatinib (P=0.036).
Full table
Tumor features
At the time of the diagnosis, the median tumor size was 4.0 (range: 0.1–55.0) cm. Of all the tumors, 615 tumors (23.9%) were ≤2 cm, 952 (37.0%) were >2 cm and ≤5 cm, 659 (25.6%) were >5 cm and ≤10 cm, and 344 (13.4%) >10 cm. In addition, the mitotic index was observed in ≤5 per 50 high-power fields (HPFs) in 1,928 cases (75.0%); in >5 and ≤10 HPFs in 342 cases (13.3%); and in >10 HPFs in 300 cases (11.7%). The median mitotic index per 50 HPFs was 3 (range: 0–254). The histological subtypes included spindle (n=2,345; 91.2%), epithelioid (n=55; 2.1%) and mixed (n=170; 6.6%) types. When classified according to the modified NIH classification, 440 patients (21.9%) were classified into the very low-risk group, 581 (28.9%) into the low-risk group, 283 (14.1%) into the intermediate-risk group, and 705 (35.1%) into the high-risk group.
Immunohistochemical expression
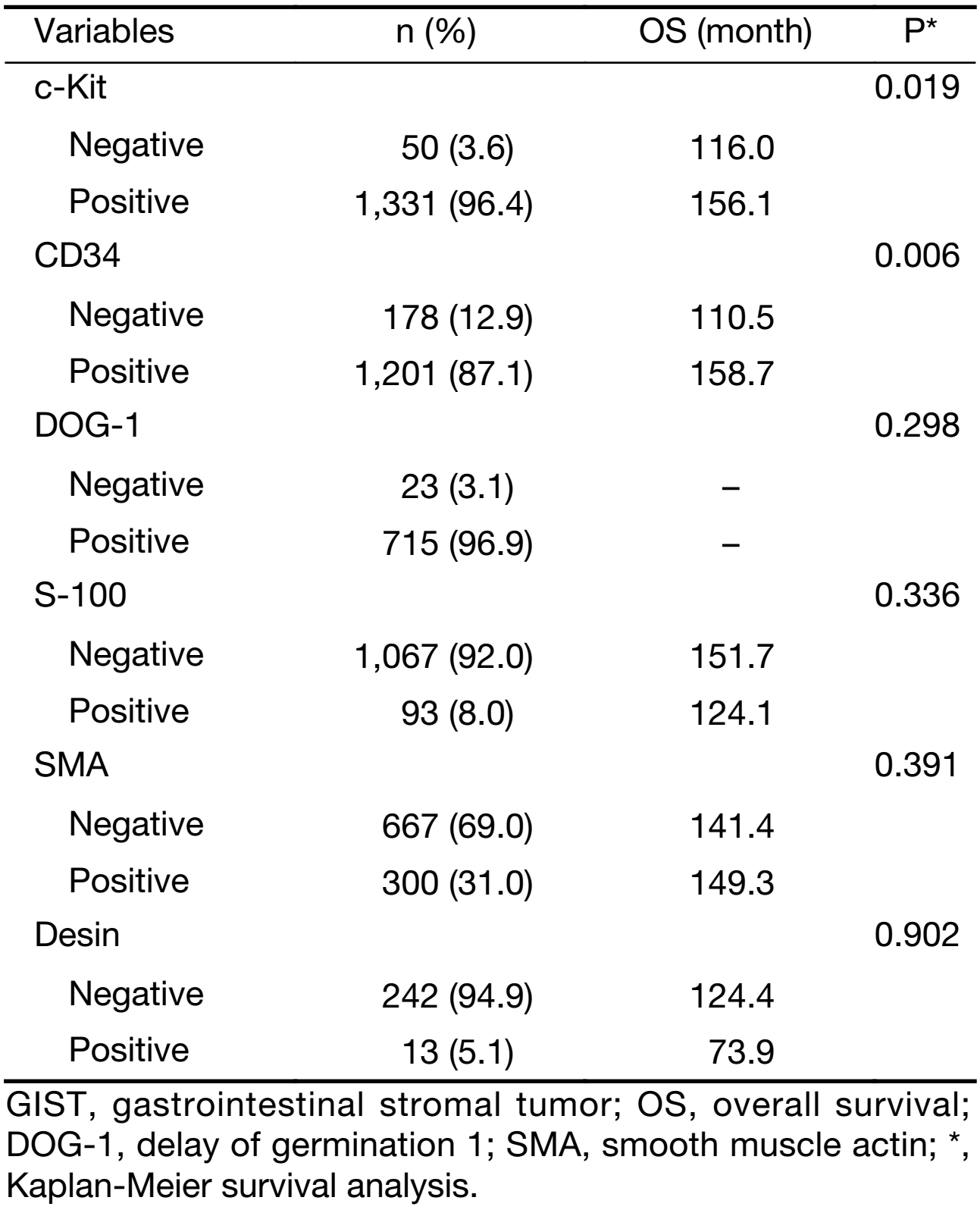
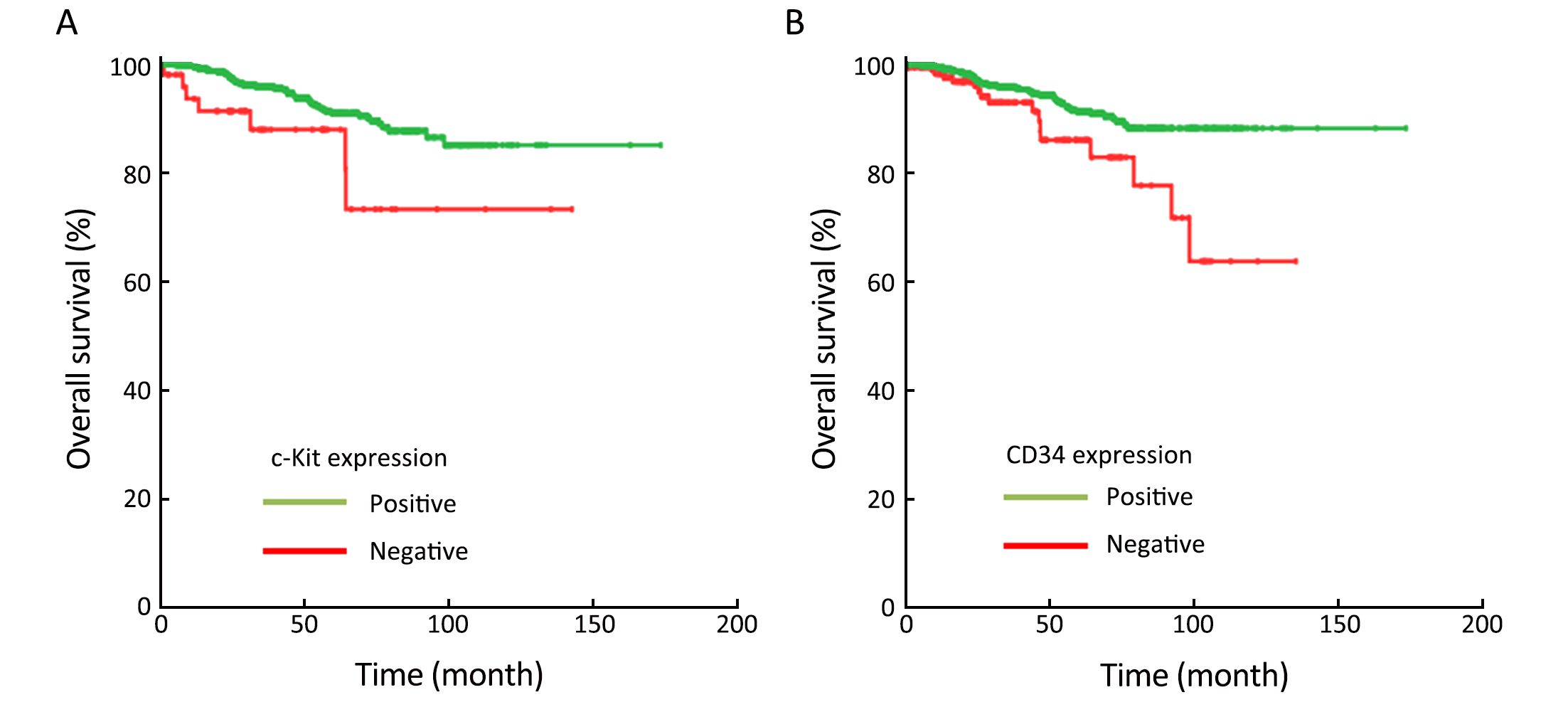
Of the 2,168 resected patients, 1,441 (66.5%) had complete follow-up data. The patient selection process is shown in Figure 1. Furthermore, the immunohistochemical features of these patients as associated with OS have been summarized in Table 2. c-Kit was immunohistochemically detected in 96.4%, CD34 in 87.1%, DOG-1 in 96.9%, S-100 in 8.0%, SMA in 31.0%, and desmin in 5.1% of the tumors tested. Kaplan-Meier survival analysis showed that c-Kit positivity and CD34 positivity correlated with favorable outcome (P=0.019; P=0.006; Figure 2), whereas the prognostic significance of other immunohistochemical markers was insignificant (All P>0.05).
Full table
Survival analysis
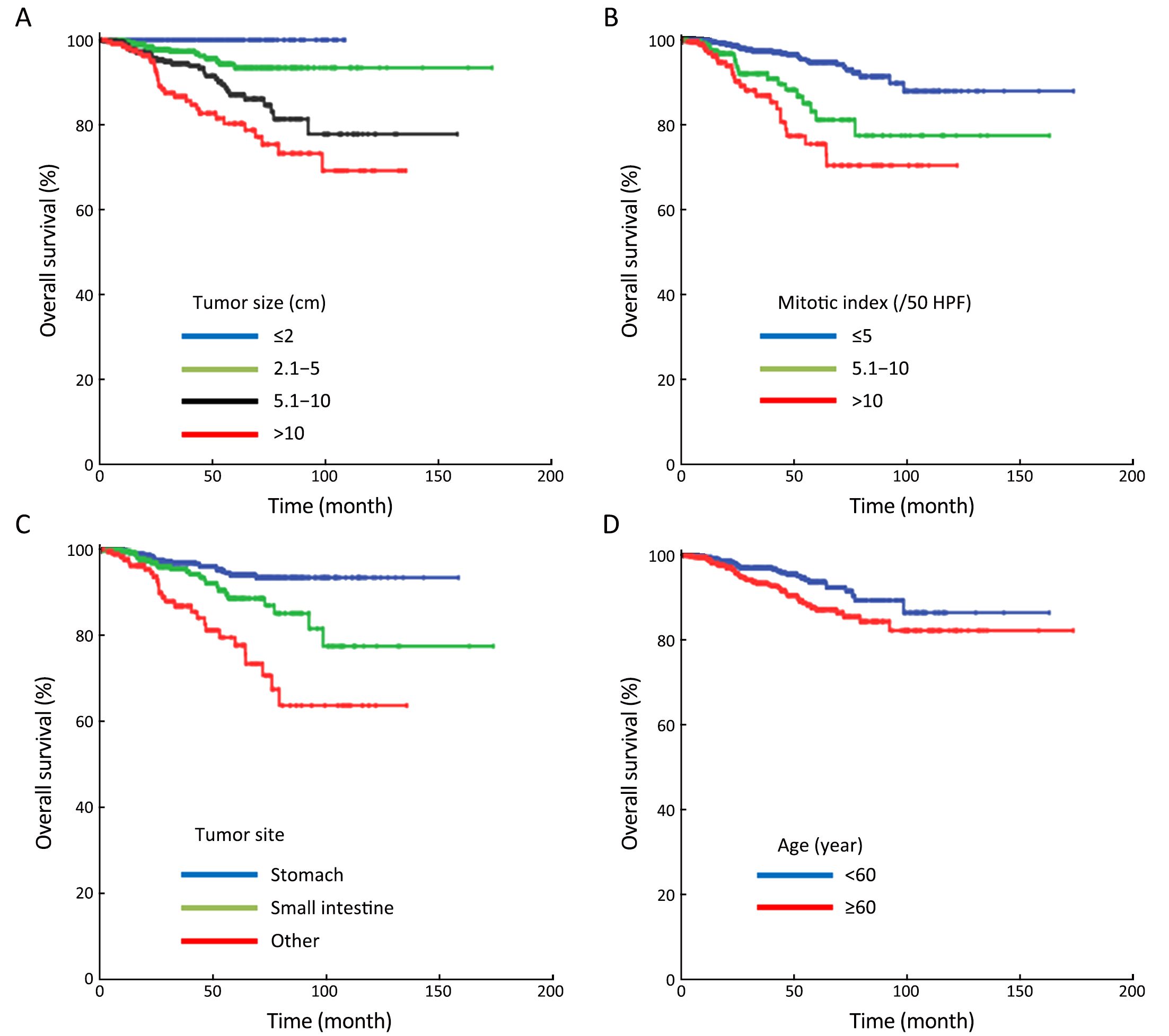
In 1,441 patients undergoing surgical resection with complete follow-up, the median follow-up period was 36 (range: 1–174) months. The 5-year survival rate by modified NIH classification was 100% in very low-risk patients, 97.5% in low-risk patients, 95.1% in intermediate-risk patients, and 80.5% in high-risk patients (Figure 3). Univariate analysis showed that age, tumor size, mitotic index, tumor site, occurrence of curative resection, postoperative imatinib, c-Kit level and CD34 level were all associated with OS (All P≤0.1). However, sex, histological subtype and occurrence of tumor rupture had no significant effect on the prognosis of patients (All P>0.1). In multivariate analysis, only age, tumor size, mitotic index, tumor site, occurrence of curative resection and postoperative imatinib were independent predictors of OS (All P<0.05;Table 3, Figure 4). In addition, 198 high-risk (39.1%) and 55 intermediate-risk (27.2%) patients were treated with surgery plus postoperative imatinib (400 mg/d). In principle, the duration of adjuvant imatinib therapy should not be less than 1 year, and patients were treated until disease progression or unacceptable side effects occurred. Of note, we found that high-risk patients benefited significantly from postoperative imatinib (P<0.001), whereas intermediate-risk patients did not (P=0.954). As shown in Figure 5, surgery plus postoperative imatinib resulted in a more favorable survival than surgery alone (OS: 96.0% vs. 64.4%; P<0.001) in high-risk patients. We further performed multivariate analyses in high-risk and intermediate-risk patients and found that the conclusion agreed with the Kaplan-Meier analysis [hazard ratio (HR): 0.201, P<0.001; HR: 1.609, P=0.714].


Full table

Discussion
Over the past decades, GIST has emerged from being a poorly recognized, treatment-resistant tumor to a well-defined and treatable tumor seen as a model of targeted oncological therapy (15-17). Even though the tumors are relatively rare, multicenter studies and consensus conferences may improve the strategies for diagnosis, treatment and follow-up of patients with GISTs (18).
In the present study, we collected data from four medical centers in China and 2,570 patients were pooled. We found that age, tumor size, mitotic index, tumor site, occurrence of curative resection and postoperative imatinib might be valuable factors in the prognostic assessment of GISTs. The integration of these parameters into prognostic models for risk-stratification is recommended. Furthermore, postoperative imatinib could improve the prognosis of high-risk patients after surgery. However, the efficacy of imatinib for intermediate-risk patients remains to be verified. These observations might have promising implications for disease monitoring and for the development of more individualized targeted therapeutic strategies.
In our study, the general clinicopathologic characteristics of GISTs were in line with previous studies (9,19,20). Recently, the first version of the Asian GIST consensus guidelines also concluded similar findings, including similar tumor features and immunohistochemical expression (21). Joensuu et al. pooled individual data from 3,067 patients and reported similar demographics of GISTs. They found that GIST patients had a slight male predominance with the median age of 64 years at the time of GIST detection (22). In addition, a review from Miettinen et al. also drew the conclusion that the median age in the major series varied between 60–65 years (3). Interestingly, our patients, with a median age of 58 years, were younger than those in Western studies. These data suggested that in our country, GISTs tended to be detected earlier. Of note, in the present study, only 1,441 (66.5%) patients had complete follow-up data for survival analysis. Due to the retrospective study design and data collection, missing data were inevitable. To maintain the representativeness of the study population, we kept as many cases as possible and used all available information for each analysis, which may have resulted in biased estimates. However, as with all retrospective studies, this parameter is difficult to verify statistically, and we must rely on the broad implications of the study. Furthermore, although there exists sample selection bias, we think it is reasonable to conclude that the missing data are difficult to alter the conclusions. Future prospective clinical studies are needed to further validate these findings.
Immunohistochemical staining of markers, such as c-Kit, CD34, SMA, S100 and DOG-1, is necessary for the accurate diagnosis of GISTs and for the differential diagnosis between GISTs and other mesenchymal tumors (9,23). Miettinen et al. revealed that c-Kit and CD34 showed diffuse strong positive expression levels in gastric GISTs and were positive in 91% and 82% of the gastric GISTs in that study, respectively (24). In addition, Kang et al. reported that the immunohistochemical stains for c-Kit, CD34 and DOG-1 were positive in 89.8%, 72.0% and 90.7% of the tumor samples, respectively (25). In fact, our findings were in accordance with these studies. Furthermore, until now, the relationship between immunohistochemical expression and prognosis has remained unclear. A recent study showed that strong DOG1 expression correlated with a worse 2-year recurrence-free survival rate, suggesting its potential ability to predict GISTs with unfavorable prognosis (26). However, another study reported that c-Kit and DOG-1 negativity might be potential prognostic factors for poor outcome in GISTs (25). In our study, although Kaplan-Meier survival analysis showed that c-Kit and CD34 positivity had potential prognostic value, none of these immunohistochemical markers were independent predictors in multivariate analysis. These findings suggested that immunohistochemical markers exerted a definite role in GIST diagnosis, whereas their prognostic role was limited.
In clinical practice, several risk-stratification systems have been proposed that take into account the established independent risk factors of tumor size, mitosis index, tumor site and occurrence of tumor rupture (27). The recently proposed modified NIH classification that incorporates those four factors is the method most widely used in clinical trials. Although it will probably remain standard for pathologists and clinicians, investigators continue to seek other prognostic factors that may help improve the clinical management of GISTs. In the current study, univariate analysis was performed on more than ten factors affecting prognosis, including age, sex and other factors. However, a multivariate analysis revealed that only age, tumor size, mitotic index, tumor site and occurrence of curative resection were independent predictors of outcome in GISTs. In fact, a study from Korea including 1,227 GIST patients also showed that age had a significant correlation with prognosis in a multivariate analysis (28). Tran et al. also found that older age was an independent predictor of mortality (20). Recently, a review of records in the Surveillance, Epidemiology and End Results (SEER) database from 2,537 patients with GISTs revealed that an age older than 65 years was a negative independent risk predictor in a multivariate analysis (29). It should be noted that age might exert potent prognostic value, which is worthy of being further verified in future studies. Furthermore, occurrence of tumor rupture did not have an independent adverse effect on outcome. Considering that there were limited cases of patients with ruptured GISTs in our study, our results should be viewed with caution.
Although the NCCN and ESMO guidelines recommend the use of adjuvant imatinib for patients with intermediate- and high-risk GISTs, there is still no valid evidence indicating whether these patients will benefit. In fact, we also found that the intermediate-risk patients had a favorable clinical outcome like the low-risk patients, meaning that only the high-risk patients would likely benefit from adjuvant imatinib. Therefore, we further investigated the clinical benefit of adjuvant imatinib in intermediate- and high-risk patients. Of note, we found that high-risk patients benefited significantly from adjuvant imatinib, whereas intermediate-risk patients did not. With our results in mind, we encourage the use of prospective multicenter randomized controlled trials to further validate our conclusions. Furthermore, whether high-risk patients are likely to benefit from prolonged treatment duration would also be of considerable interest to study in the future (30).
Our study had some limitations. Several recent studies focusing on the mutation types of GISTs reported that they might add important prognostic information for risk assessment (31-33). Unfortunately, we lacked the data to further investigate this idea. Furthermore, to maintain an unselected consecutive cohort, we did not exclude a small number of GISTs with a diameter of 1 cm or less, though these tumors usually had very low malignant potential (34). Finally, some intermediate- and high-risk patients were not treated with a standard dose and duration of imatinib, which may have confounded the results.
Conclusions
We found that age, tumor size, mitotic index, tumor site, occurrence of curative resection and postoperative imatinib might be important independent prognostic indicators in GISTs. The integration of these parameters into future stratification schemes may improve the accuracy of risk assessments. Furthermore, considering the potential side-effects and the financial costs of treatment, the efficacy of imatinib for intermediate-risk patients needs to be verified.
Acknowledgements
The authors thank all of the people who helped with this study. This work was supported by the National Science Foundation of China (Grant No. 81372474, 81602061) and Science and Technology Program of Guangzhou (No. 2014J4100179).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973–83. [PubMed] DOI:10.1016/s0140-6736(13)60106-3
- Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J Gastrointest Oncol 2017;8:466–73. [PubMed] DOI:10.21037/jgo.2016.09.15
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am 2013;42:399–415. [PubMed] DOI:10.1016/j.gtc.2013.01.001
- Wang C, Zheng B, Chen Y, et al. Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs. Chin J Cancer Res 2013;25:63–70. [PubMed] DOI:10.3978/j.issn.1000-9604.2012.12.01
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25:1107–13. [PubMed] DOI:10.1200/jco.2006.09.0183
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281–93. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.04.01
- Eisenberg BL, Trent JC. Adjuvant and neoadjuvant imatinib therapy: current role in the management of gastrointestinal stromal tumors. Int J Cancer 2011;129:2533–42. [PubMed] DOI:10.1002/ijc.26234
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 2002;10:81–9. [PubMed] DOI:10.1177/106689690201000201
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83. [PubMed]
- Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;97:1854–9. [PubMed] DOI:10.1002/bjs.7222
- Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007;14:2018–27. [PubMed] DOI:10.1245/s10434-007-9377-9
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411–9. [PubMed] DOI:10.1016/j.humpath.2008.06.025
- Reichardt P, Blay JY, Boukovinas I, et al. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol 2012;23:2776–81. [PubMed] DOI:10.1093/annonc/mds198
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era — a population-based study in western Sweden. Cancer 2005;103:821–9. [PubMed] DOI:10.1002/cncr.20862
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4. [PubMed]
- Blay JY. A decade of tyrosine kinase inhibitor therapy: Historical and current perspectives on targeted therapy for GIST. Cancer Treat Rev 2011;37:373–84. [PubMed] DOI:10.1016/j.ctrv.2010.11.003
- Renouf DJ, Wilson L, Blanke CD. Successes and challenges in translational research: the development of targeted therapy for gastrointestinal stromal tumours. Clin Cancer Res 2009;15:3908–11. [PubMed] DOI:10.1158/1078-0432.ccr-08-1622
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3–14. [PubMed] DOI:10.1007/s10120-015-0526-8
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39–46. [PubMed] DOI:10.1016/j.canep.2015.10.031
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162–8. [PubMed] DOI:10.1111/j.1572-0241.2005.40709.x
- Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155–66. [PubMed] DOI:10.4143/crt.2016.187
- Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634–42. [PubMed] DOI:10.1200/jco.2014.57.4970
- Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477–89. [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52–68. [PubMed]
- Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat 2010;42:135–43. [PubMed] DOI:10.4143/crt.2010.42.3.135
- Rizzo FM, Palmirotta R, Marzullo A, et al. Parallelism of DOG1 expression with recurrence risk in gastrointestinal stromal tumors bearing KIT or PDGFRA mutations. BMC Cancer 2016;16:87. [PubMed] DOI:10.1186/s12885-016-2111-x
- Yanagimoto Y, Takahashi T, Muguruma K, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer 2015;18:426–33. [PubMed] DOI:10.1007/s10120-014-0386-7
- Cho MY, Sohn JH, Kim JM, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci 2010;25:853–62. [PubMed] DOI:10.3346/jkms.2010.25.6.853
- Woodall CE 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 2009;144:670–8. [PubMed] DOI:10.1001/archsurg.2009.108
- Casali PG, Fumagalli E, Gronchi A. Adjuvant therapy of gastrointestinal stromal tumors (GIST). Curr Treat Options Oncol 2012;13:277–84. [PubMed] DOI:10.1007/s11864-012-0198-0
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563–70. [PubMed] DOI:10.1200/jco.2013.51.2046
- Zong L, Chen P. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumors: a meta-analysis. World J Surg Oncol 2014;12:71. [PubMed] DOI:10.1186/1477-7819-12-71
- Wozniak A, Rutkowski P, Piskorz A, et al. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann Oncol 2012;23:353–60. [PubMed] DOI:10.1093/annonc/mdr127
- Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110–8. [PubMed] DOI:10.1002/cncr.30239