A nomogram to predict adjuvant chemotherapy recommendation in breast cancer patients with intermediate recurrence score
Introduction
Over the past decade, prognostic assays based on multigene expression have been used in breast cancer to estimate the residual risk of recurrence following surgery to aid appropriate decisions regarding chemotherapy. The most widely used test is the 21-gene breast recurrence score (RS) assay, which is a reverse transcriptase polymerase chain reaction (RT-PCR) assay for 5 reference genes and 16 cancer-related genes, grouped into cell proliferation, invasion, human epidermal growth factor receptor 2 (HER2) and estrogen receptor (ER) categories (1). The assay was initially validated to predict the likelihood of a 10-year recurrence risk and chemotherapy benefit in ER-positive, HER2-negative, lymph node (LN)-negative early breast cancer (EBC) patients (2-4).
Although originally validated and recommended for LN-negative diseases, retrospective evidence accrued thereafter also demonstrated its prognostic utility among selected patients with minimal LN involvement (1–3 positive nodes) (5,6). As a consequence, the 2015 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Breast Cancer have incorporated the RS test into a clinical routine for LN-positive patients, specifically noting that this test can be considered for selected women with 1–3 involved ipsilateral axillary nodes, to guide the addition of combination chemotherapy to standard hormone therapy (7).
Based on the RS assay results, patients are traditionally categorized into low- (RS<18), intermediate- (RS 18–30) and high-risk (RS>30) groups following the retrospective analysis of NSABP B-14 trial (1). Patients in the high-risk category benefit significantly from adjuvant chemotherapy while those in the low-risk category do not. However, the advantage of chemotherapy in patients with intermediate RS is still uncertain (2). Currently, a prospective TAILORx trial is underway to evaluate the ability of RS to optimize therapeutic decisions for this subset of patients (RS 11–25), by randomizing enrolled participants with RS≤25 to receive hormonal therapy either alone or in combination with chemotherapy (3). WSG Plan B trial was the first to evaluate the efficacy of adjuvant chemotherapy (4 cycles of epirubicin plus cyclophosphamide followed by 4 cycles of docetaxel versus 6 cycles of docetaxel plus cyclophosphamide) in genomically intermediate/high-risk HER2-negative EBC. The final analysis of the trial showed an excellent 5-year disease-free survival (DFS) in intermediate RS tumors, which suggested potential overtreatment by chemotherapy (8). At present, treatment decision for patients with intermediate RS is multifactorial, and is based on both tumor characteristics (tumor grade and size, molecular subtype and LN status) and patients’ characteristics (age, menopausal status, and performance status) (9). However, the results of multivariate analysis of adjuvant chemotherapy recommendation (ACR) are usually expressed as odds ratios (OR), which make it difficult to apply and calculate the probability of ACR for a specific patient.
To date, no statistical models have been reported to predict the likelihood of ACR in patients with intermediate RS. Nomograms have gained popularity in clinical practice and have been applied in individualized prognosis. They are statistical tools that provide the overall probability of a specific outcome for an individual patient. Factors associated with a defined event are incorporated in the nomogram and the calculated probability of the event occurrence is provided in graphical formats (10,11). This study aimed to identify clinicopathological predictors of ACR in breast cancer patients with intermediate RS, and to establish a nomogram for predicting the probability of ACR. This model might aid in avoiding potential overtreatment by chemotherapy in patients indicated with low ACR probability.
Materials and methods
Patients
EBC patients who underwent surgical treatment between January 2014 and December 2016 at the Shanghai Ruijin Hospital were retrospectively reviewed. The inclusion criteria were as follows: 1) female; 2) pathologically diagnosed invasive ductal carcinoma (IDC); 3) pT1b–T2; 4) pN0, N1mi or N1 [according to the MINDACT trial micrometastases measuring 0.2 to 2 mm were considered to be LN-positive in the following analysis (12)]; 5) ER and/or progesterone receptor (PR) positive tumors determined by immunohistochemistry (IHC); 6) HER2 negative; and 7) adequate formalin-fixed paraffin-embedded tissue available to perform the 21-gene assay and with an RS result of 11–30. The exclusion criteria included: 1) T1a, T3 or T4 tumor; 2) metastatic breast cancer; or 3) previous neoadjuvant treatment. The baseline data including age, tumor characteristics (tumor size, LN status, and histological grade: ER, PR, Ki67) and surgical information were retrieved.
This retrospective study was approved by the Ethical Committees of the Shanghai Ruijin Hospital. The results of this study do not affect the treatment decision of any patient enrolled. The written informed consent was obtained from the patients prior to data collection.
Pathological evaluation
Tumors were classified histologically according to the World Health Organization Classification of Tumors (13). Histological grade was evaluated according to the Elston and Ellis scoring system (14). Positive staining for ER/PR was defined as nuclear staining in ≥1% of the tumor cells (15). Negative HER2 status was considered as 0 to 1+ by IHC or negative on fluorescence in situ hybridization (FISH) (16). Patients were subdivided into two different molecular phenotypes (luminal A and luminal B subtypes) according to the 2013 St. Gallen Expert Consensus (17).
Analysis of RS
The RS was analyzed from formalin-fixed, paraffin-embedded tissue as previously described (1). Briefly, micron tissue sections stained with hematoxylin and eosin (HE) were reviewed by a pathologist (XC Fei). A tumor-rich area in the tumor block was marked and manually microdissected with clean blades. RNA was extracted according to standard operating procedure for the 21-gene RS assay, and was subjected to gene-specific reverse transcription followed by quantitative RT-PCR reactions in 96 well plates using Applied Biosystems (Foster City, CA) 7500 Real-Time PCR System. Expression of each gene was measured in triplicate, and normalized relative to a set of five reference genes. Reference-normalized expression measurements range from 0 to 15, with a 1-unit increase reflecting approximately a two-fold increase in RNA. The RS, ranging from 0 to 100, was derived from the reference-normalized expression measurements for the 16 cancer-related genes (1). In order to further evaluate the effect of the cutoff point chosen in the retrospective analysis of the NSABP B-20 trial (RS results: 18–30) (2) and the ongoing prospective TAILORx trial (RS results: 11–25) (3), the enlarged intermediate category was defined as 11 to 30 in our study and was divided into three subcategories of 11 to 17, 18 to 25 and 26 to 30.
Outcomes
The outcome of nomogram was the probability of ACR in patients with intermediate RS. As the standard of care, the actual ACT decision was determined by multidisciplinary team (MDT) model, which is a critical part of the clinical practice in the management of complex malignancies. The make-up of MDT for breast cancer includes breast surgeons, medical oncologists, radiation oncologists, pathologists, and breast radiologists, as represented in the always popular “community tumor board”. Breast cancer nurses (BCN) also have an important role in this model, providing information, psychological support, advocacy, and coordinating care through the pathway (18,19).
The operation flow of MDT in this study is as below: Following surgery and pathology assessment, upon receipt of the RS results, BCN coordinate a meeting of the MDT team members at a given time to discuss the given patients recently operated. After briefly introducing the patient’s clinicopathological information by interns, the members vote for adjuvant therapies. The staff then discusses mainly the controversies or inconsistencies in treatment strategies based on the results of the primary vote. Finally, a secondary vote is completed, and the treating doctor informs the patients of the final decision.
Nomogram construction and validation
Univariate and multivariate logistic regression analyses were used to screen the predictors (20). Variables that were statistically significant (P<0.05) in the univariate logistic analysis of the training set were included in the multivariate logistic regression analysis, which was performed to screen independent predictors for ACR. Independent predictors (P<0.05 in the multivariate logistic regression analysis) were included in the nomogram construction. Hosmer and Lemeshow test was applied to assess the goodness of fit of the model, and P>0.05 indicated a good fit (21). The OR and 95% confidence interval (95% CI) were also calculated.
Evaluation of nomogram performance
The nomogram was validated internally in the training set and externally in the validation set. The internal validation was performed by a calibration method and receiver-operating characteristic (ROC) curves. The area under the curve (AUC) was calculated. The external validation was performed by calculating the AUC. The calibration plot with bootstrapping was used to illustrate the association between the actual probability and the predicted probability (22). Statistical differences between different AUCs were investigated by the DeLong method (23). All the statistical analyses and graphics were performed with the IBM SPSS Statistics (Version 20.2; IBM Corp., New York, USA) and R software (Version 2.11.1; R Foundation for Statistical Computing, Vienna, Austria). P<0.05 was considered statistically significant.
Results
Patient characteristics and predictors for ACR
The overall data from 504 breast cancer patients with intermediate RS were retrospectively analyzed. The mean age of the patients enrolled was 57 (range, 29–88) years. The patients enrolled were randomized 3:1 and divided into a training set (n=378) and a validation set (n=126). Of the 504 patients, 255 (50.6%) were recommended for chemotherapy following the MDT discussion. The clinicopathological characteristics and the univariate logistic regression analysis of the total population, the training set and the validation set are shown in Table 1. Patients with younger age (≤50 years), larger tumor size, higher histological grade, LN involvement, luminal B subtype or higher RS were more likely to have ACR than the other patients (P<0.05). No significant difference in the ACR rate was observed among patients with different types of breast surgery (P=0.092). Univariate analysis of the training set and the validation set showed similar results compared with patients in the total population.
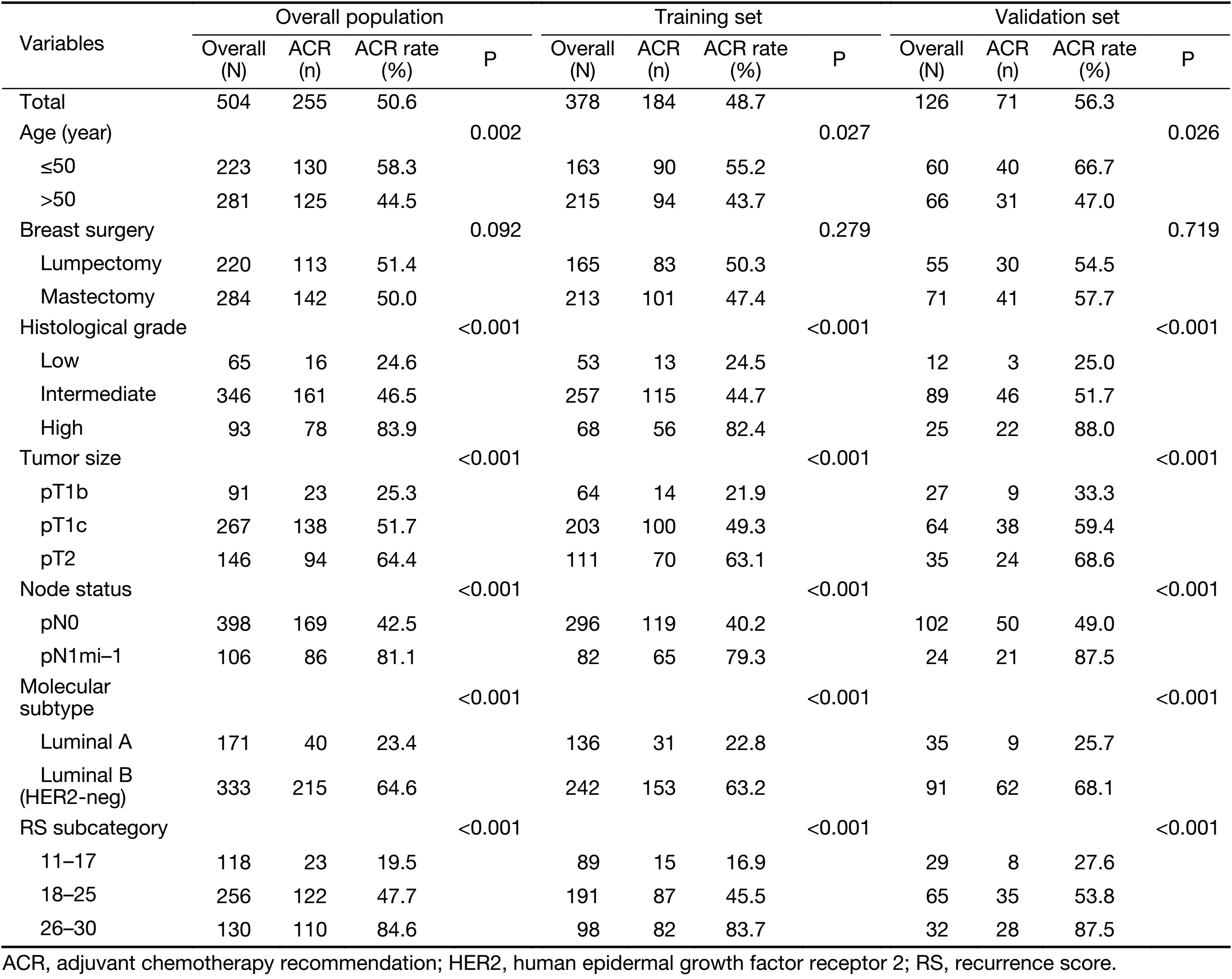
Full table
Associations between candidate predictive indicators and ACR were evaluated using multivariate logistic regression analysis. Predictors that were statistically significant (P<0.05) in the univariate logistic analysis were included in the multivariate logistic regression analysis. The results of the analysis indicate that age, histological grade, tumor size, LN status, molecular subtype and RS are independent predictors associated with ACR (Table 2).
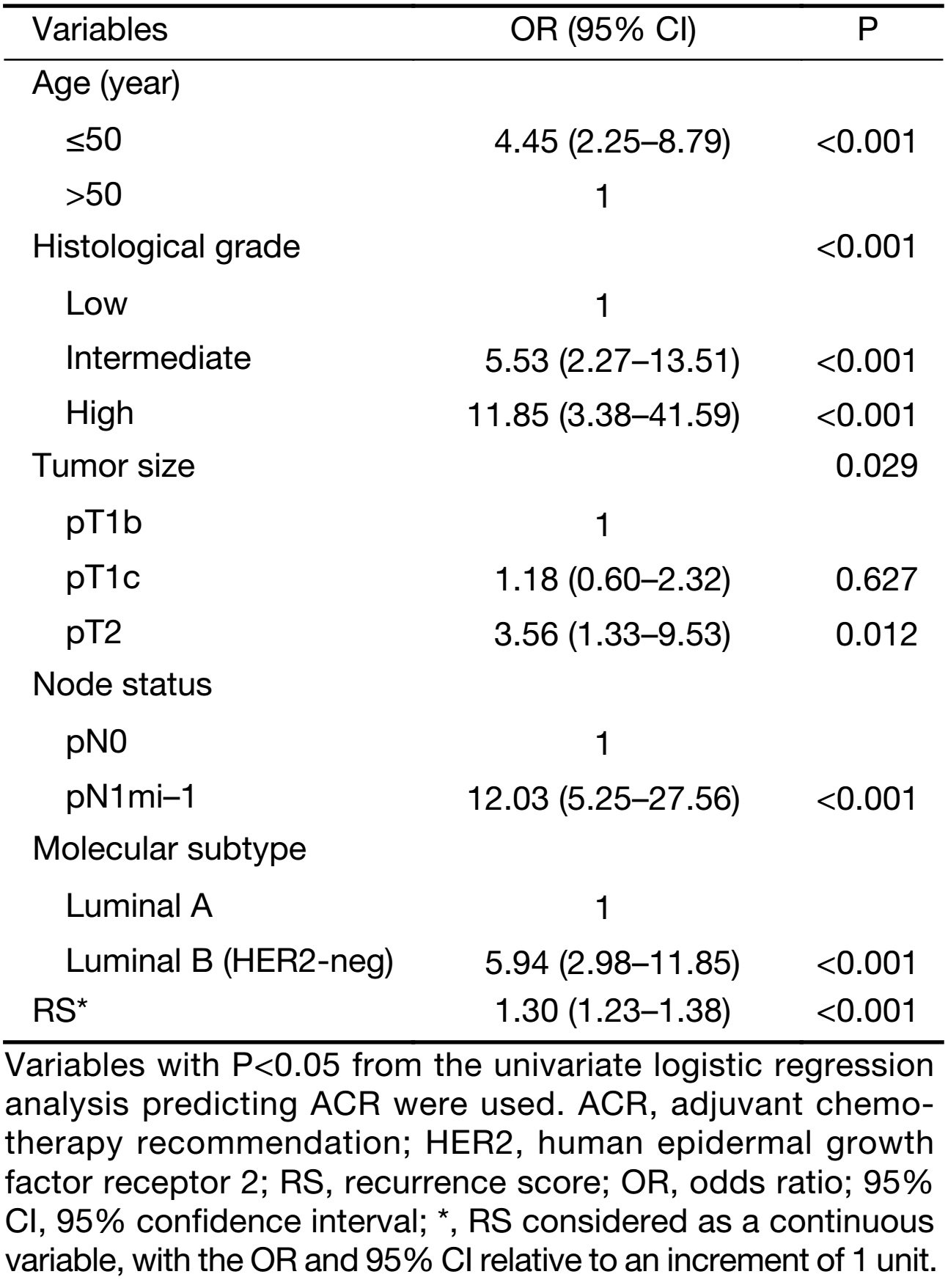
Full table
Construction and validation of nomogram
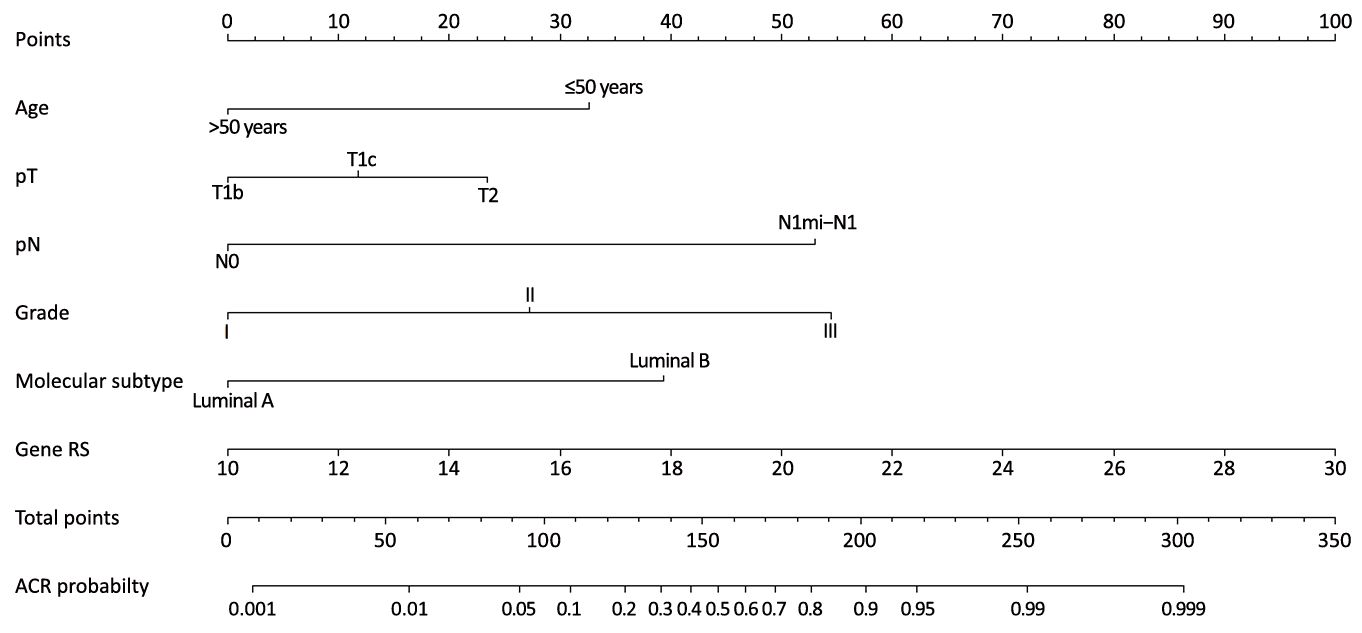
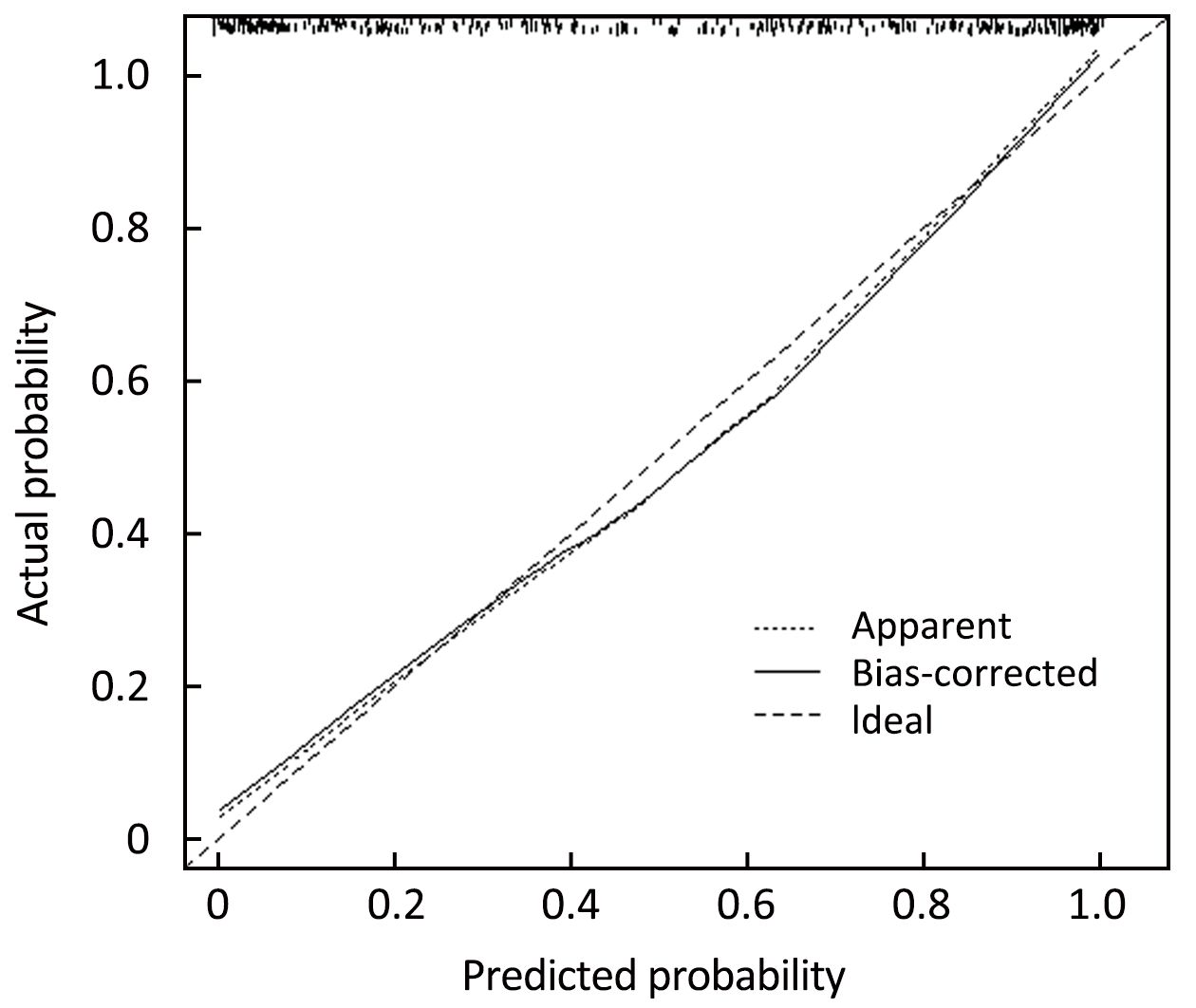
Independent predictors identified in the multivariate logistic regression analysis (P<0.05), including age, tumor size, histological grade, LN status, molecular subtype and RS were utilized to construct the nomogram. The total points were calculated by summing up the points for each variable (top plotting scale). The ACR probability was subject to the total points (bottom plotting scale). The P-value for the Hosmer and Lemeshow test was 0.286, indicating that the model had a good fit. The final nomogram is shown in Figure 1. The calibration of the nomogram was performed internally by a calibration plot with bootstrap sampling (n=1,000) (Figure 2). The calibration plot of an accurate model may fall along the 45-degree line. Our bias-corrected curve was close to the ideal curve, which indicated that the nomogram was well calibrated. Next, the ROC was calculated to validate the nomogram internally in the training set (Figure 3A) and externally in the validation set (Figure 3B). The AUC was 0.905 (95% CI: 0.876–0.934) in the training set and 0.883 (95% CI: 0.824–0.942) in the validation set. The difference between the two AUCs was not statistically significant (P=0.132), illustrating that the predicted and observed ACR probabilities were in good concordance, and the goodness of fit of the nomogram was favorable.
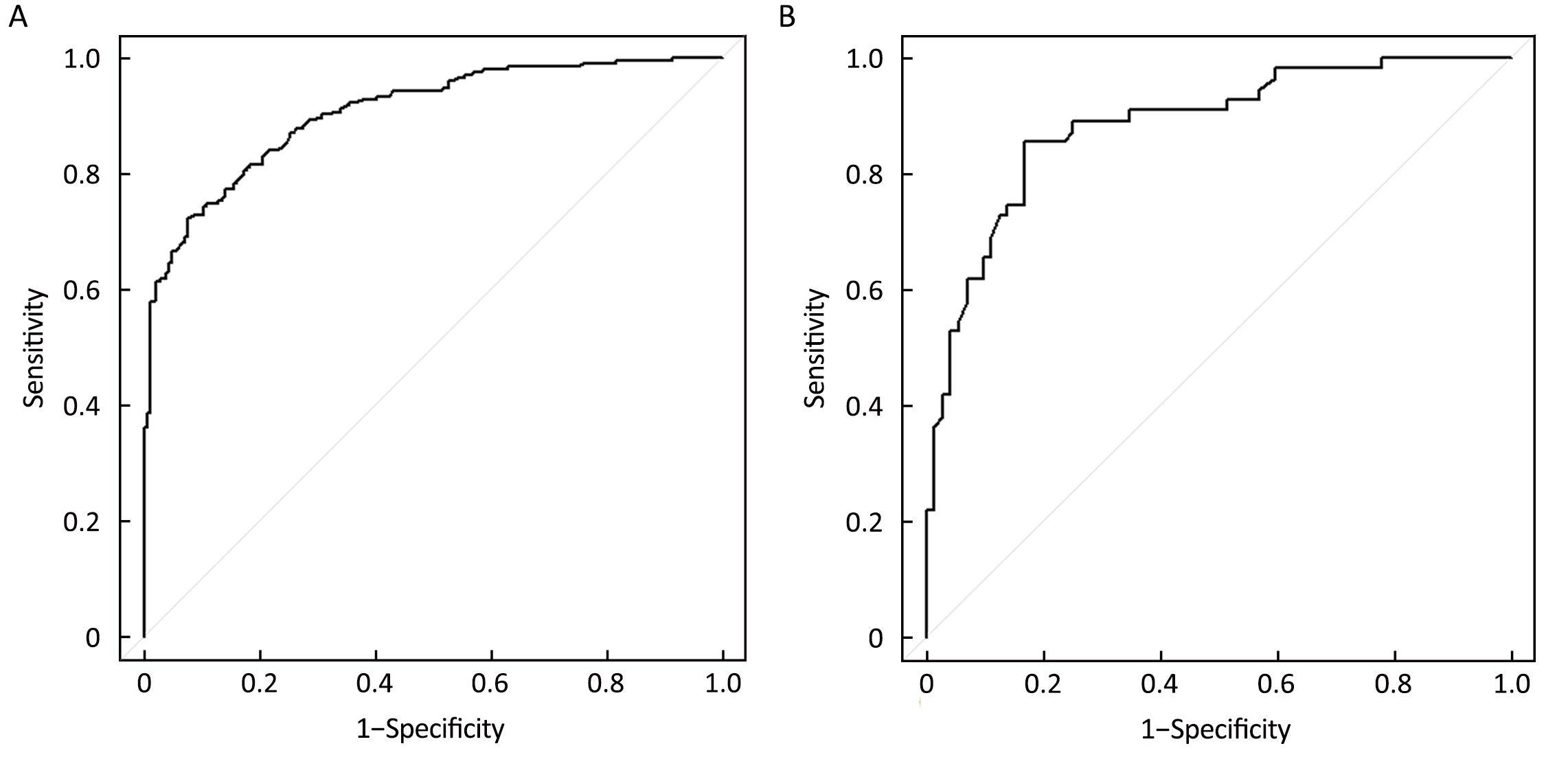
The optimal cutoff values for the training and validation sets were 0.61 (sensitivity: 85.5%; specificity: 83.3%; positive predictive value: 83.7%; negative predictive value: 85.2%; accuracy: 84.4%) and 0.67 (sensitivity: 89.1%; specificity: 75.0%; positive predictive value: 78.1%; negative predictive value: 87.3%; accuracy: 82.1%), respectively.
Discussion
Our results demonstrate that younger age, larger tumor size, higher histological grade, LN involvement, luminal B subtype and higher RS are associated with high ACR rates in patients with intermediate RS. The predictive value of each of the above-mentioned indicators was relatively poor, and we, therefore, combined the significant predictive indicators based on multivariate logistic regression analysis of the training set and developed a nomogram for evaluating the probability of ACR in this subset of patients. Furthermore, we validated the effectiveness of the nomogram using the second data set. The AUC values for the training and validation sets suggested that the nomogram performed well. We also proposed a cut-off value for ACR to help avoid potential overtreatment by chemotherapy. We believe that this user-friendly nomogram will be useful for risk assessment and could be the basis for individualized risk-adaptive therapy.
To date, few predictive models have been reported for breast cancer patients with intermediate RS. In 2011, Tang et al. (24) developed an online Recurrence Score Pathology-Clinical (RSPC) calculator, which is used in node-negative patients and combines RS with clinicopathological variables including age, tumor size, grade, and planned adjuvant hormonal therapy. RSPC calculator is likely to have the greatest clinical utility in patients with intermediate RS by refining assessments of recurrence risk where RS and traditional measures are discordant and by reducing the number of patients classified as intermediate risk. In this study, the gene signature (GS) was combined into a nomogram on the basis of five important clinicopathological factors for better discrimination power (AUC=0.860 without RS). These results indicated that integration of clinicopathological factors with molecular features could enhance the prognostic power of risk assessments.
As expected, in our study, higher odds of recommending chemotherapy were associated with younger age, larger tumor size, higher tumor grade, more nodes involvement and luminal B subtype. It is noteworthy that the RS result is an independent predictor of ACR in our study. Moreover, when the intermediate group was further subdivided, those with scores of 26 to 30 had a significant chance of receiving a chemotherapy recommendation than those with scores of below 25 (P<0.05). Our findings were concordant with those of Jasem et al., who evaluated the effect of RS cutoff point on chemotherapy decision. It was indicated that, when divided based on the cutoff point of 25 adopted by the TAILORx trial, those with an RS of 18 to 25 had significantly lower odds of chemotherapy recommendation compared with those with an RS of 26 to 30 (OR=0.32; 95% CI: 0.26–0.40) (25). These results suggested that clinicians evaluate the RS as a continuous parameter and do not consider all intermediate RS patients as having the same risk of recurrence.
Of note, in our study 20% of the enrolled patients are node-positive, of whom 18.9% were not recommended for chemotherapy. This finding supported the evidence that not all patients with the LN-positive disease have aggressive characteristics. In this context, the prospective RxPONDER trial was designed to test whether the difference of chemotherapy compared with no chemotherapy depends directly on RS score in patients with one to three positive axillary nodes, thus determining the optional cut point for recommending chemotherapy (26). Accrual is currently underway and outcomes are not yet available. However, in clinical practice, routine administration of adjuvant chemotherapy is still strongly proposed for women with node-positive breast cancer regardless of their tumor biology (27).
One strength of our study is that this nomogram can be used for predicting ACR with the combined TAILORx-trial (using a cutoff of 11 and 25) and the original cut-off score model (using a cutoff of 18 and 30). This nomogram, therefore, accommodates for the differences in the intermediate risk scoring results currently in use. We believe that this option gives our study an advantage over other studies which did not use the combination of original and TAILORx-trial cut-off values.
Lastly, our model is imperfect. This study was limited by the relatively small number of cases associated with the retrospective nature of the study. The data were collected from a single institution, and selection bias existed even though consecutive patients were included and eligibility criteria were performed to minimize the bias. Further prospective studies are therefore warranted to validate the suitability of this model for clinical practice.
Conclusions
The present study constructed a nomogram based on routine clinicopathological parameters and a GS (21-gene RS). This tool might help in predicting the probability of ACR in patients with intermediate RS and thus can assist clinicians in making adjuvant chemotherapy decisions.
Acknowledgements
The authors are grateful to Tienan Feng (Clinical Research Center, Shanghai Jiao Tong University School of Medicine, China) for his excellent data management.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26. [PubMed] DOI:10.1056/NEJMoa041588
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34. [PubMed] DOI:10.1200/JCO.2005.04.7985
- Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14. [PubMed] DOI:10.1056/NEJMoa1510764
- Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist 2007;12:631–5. [PubMed] DOI:10.1634/theoncologist.12-6-631
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65. [PubMed] DOI:10.1016/S1470-2045(09)70314-6
- Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34. [PubMed] DOI:10.1200/JCO.2009.24.4798
- National Comprehensive Cancer Network (2015) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer (Version 3. 2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Harbeck N, Gluz O, Clemens MR, et al. Prospective WSG phase III PlanB trial: Final analysis of adjuvant 4xEC→4x doc vs. 6x docetaxel/cyclophosphamide in patients with high clinical risk and intermediate-to-high genomic risk HER2-negative, early breast cancer . J Clin Oncol 2017;35(suppl 15S):abstract 504.
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies — improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46. [PubMed] DOI:10.1093/annonc/mdv221
- Orucevic A, Bell JL, McNabb AP, et al. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 2017;163:51–61. [PubMed] DOI:10.1007/s10549-017-4170-3
- Zheng Z, Zhang Y, Zhang L, et al. Nomogram for predicting lymph node metastasis rate of submucosal gastric cancer by analyzing clinicopathological characteristics associated with lymph node metastasis. Chin J Cancer Res 2015;27:572–9. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.12.06
- Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29. [PubMed] DOI:10.1056/NEJMoa1602253
- Tavassoli FA, Devilee P (Eds.): World Health Organization Classification of Tumours. Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Lyon: IARC, 2003.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 2002;41:154–61. [PubMed]
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. [PubMed] DOI:10.1200/JCO.2009.25.6529
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [PubMed] DOI:10.1200/JCO.2013.50.9984
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–23. [PubMed] DOI:10.1093/annonc/mdt303
- Boughey JC, Dietz J. Providing the best care for patients with breast cancer through use of the multidisciplinary team. Ann Surg Oncol 2014;21:3163–5. [PubMed] DOI:10.1245/s10434-014-3958-1
- Wanchoo P, Larrison C, Rosenberg C, et al. Identifying educational needs of the multidisciplinary cancer team in the treatment of metastatic breast cancer. J Natl Compr Canc Netw 2017;15:205–212. [PubMed] DOI:10.6004/jnccn.2017.0021
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173–80. [PubMed] DOI:10.1016/S1470-2045(14)71116-7
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edition. New York: Wiley, 2005.
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. 1st edition. New York: Chapman & Hall, 1993.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed]
- Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol 2011;29:4365–72. [PubMed] DOI:10.1200/JCO.2011.35.3714
- Jasem J, Fisher CM, Amini A, et al. The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Canc Netw 2017;15:494–503. [PubMed] DOI:10.6004/jnccn.2017.0049
- Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotype DX-guided management for women with breast cancer involving lymph nodes. Contemporary Clinical Trials 2013;34:1–9. [PubMed] DOI:10.1016/j.cct.2012.09.003
- Hayes DF. Targeting adjuvant chemotherapy: a good idea that needs to be proven!. J Clin Oncol 2012;30:1264–7. [PubMed] DOI:10.1200/JCO.2011.38.4529
