Long-term outcomes in patients with ypT0 rectal cancer after neoadjuvant chemoradiotherapy and curative resection
Introduction
In China, colorectal cancer is the fourth most common cancer with new cases of 60,900, and its incidence is increasing (1). Currently, the standard management for locally advanced rectal cancer (LARC), clinically staged as T3, T4, and/or N+, is neoadjuvant chemoradiotherapy (NCRT) combined with curative resection (2,3). NCRT may induce tumor down-sizing and down-staging, and good responders are thought to achieve a pathological complete response (pCR) which means the absence of residual viable tumor cells in the surgical resection specimens (ypT0N0). The incidence of pCR has been reported in 10%–27% of patients, who show excellent survival rates with 5-year disease-free survival (DFS) of 83%–96% (4-8). In principle, curative surgery with total mesorectal excision (TME) is still routinely carried out in LARC patients after NCRT, regardless of the clinical response. However, in view of the potential morbidity and mortality, and the permanent stoma associated with curative resection, there has been an increasing interest in recent years in a watchful waiting strategy or local excision (LE) of rectal cancer for patients who show a good response to NCRT (9-13).
However, non-operative management or LE does not address metastatic lymph nodes in the mesorectum. Although complete pathological response of the primary tumor (ypT0) can achieve excellent outcomes, there is still the risk of metastatic lymph nodes in the mesorectum associated with tumor recurrence (14). Several reports have shown that the incidence of metastatic lymph nodes in ypT0 disease ranges from 6.6% to 16%, and that ypN+ status is a significantly independent risk factor correlated with decreased 5-year DFS and overall survival (OS) (15-20).
Therefore, we conducted this study to assess the incidence of lymph node metastases in patients with ypT0 rectal cancer treated with NCRT and curative resection, and to explore risk factors associated with survival.
Materials and methods
Patient cohort
We conducted a retrospective consecutive cohort study of patients with rectal cancer who had undergone curative resection following NCRT at the Cancer Hospital, Chinese Academy of Medical Sciences, between 2005 and 2014. Eligible patients were selected according to the following inclusion criteria: 1) had pathologically confirmed rectal cancer; 2) were clinically staged as T3, T4, and/or N+; 3) were classified on pathological examination as ypT0 (no viable tumor cells in the rectal wall) after NCRT and curative resection; 4) no distant metastases; and 5) had undergone R0 resection. Patients were excluded if they had other malignancies or had a previous history of malignant disease or recurrence, or had undergone local resection. The study was approved by the Institutional Review Board Committee of the Cancer Hospital, Chinese Academy of Medical Sciences.
Treatment
Colonoscopic biopsy was performed for all patients before NCRT to confirm the pathology and to classify differentiation of tumor into the following categories: well, moderately, poorly differentiated, and unknown. The clinical stage prior to NCRT was decided by a digital rectal examination, rectal magnetic resonance imaging (MRI), and/or endorectal ultrasound (EUS), and pulmonary and abdominopelvic contrast-enhanced computed tomography (CT) scans. Patients were treated with NCRT with a total radiotherapy dose of 42 to 50 Gy in 21 to 25 fractions with concurrent chemotherapy (single-agent capecitabine with or without oxaliplatin). Operations were generally performed 6 to 8 weeks following the completion of NCRT by an experienced colorectal surgical team according to the principles of the TME technique. All patients were generally recommended to receive adjuvant chemotherapy. A pathology assessment by two gastrointestinal pathologists was performed on all surgical specimens, and they were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system.
Follow-up
All patients were followed up at 3-month intervals for 2 years, at 6-month intervals for the next 3 years, and annually thereafter. The follow-up examinations included a clinical history, physical examination, serum carcinoembryonic antigen (CEA), stool occult blood text, chest X-ray, abdominal ultrasonography, colonoscopy, abdominopelvic CT or MRI, and positron emission tomography (PET) scanning if available. Determination of recurrence was made by clinical and radiological examinations or by histological confirmation. All patients included in this study were contacted at the time of the study to confirm their survival and recurrence status. The end point of the follow-up was 1 September 2017.
Statistical analysis
Categorical variables were reported as number (frequency); and quantitative variables were reported as median (interquartile range; IQR) and x±s and compared by the Wilcoxon rank sum test. Recurrence-free survival (RFS) was defined as the time between the date of surgery and the first tumor recurrence (local or distant metastases), and OS was defined as the time between the date of surgery and the date of death from any cause or the last follow-up. Survival was assessed using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox regression analyses were performed to verify the effects of prognostic factors found with P<0.2 in univariate analyses on RFS and OS; however, the factor “Tumor differentiation” was excluded to test its value on OS because tumor differentiation was available for just 59.3% of patients, which might decease the statistical power. All tests were two-sided, and a P-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed with IBM SPSS software (Version 22.0; IBM Corp., New York, USA).
Results
Patient characteristics
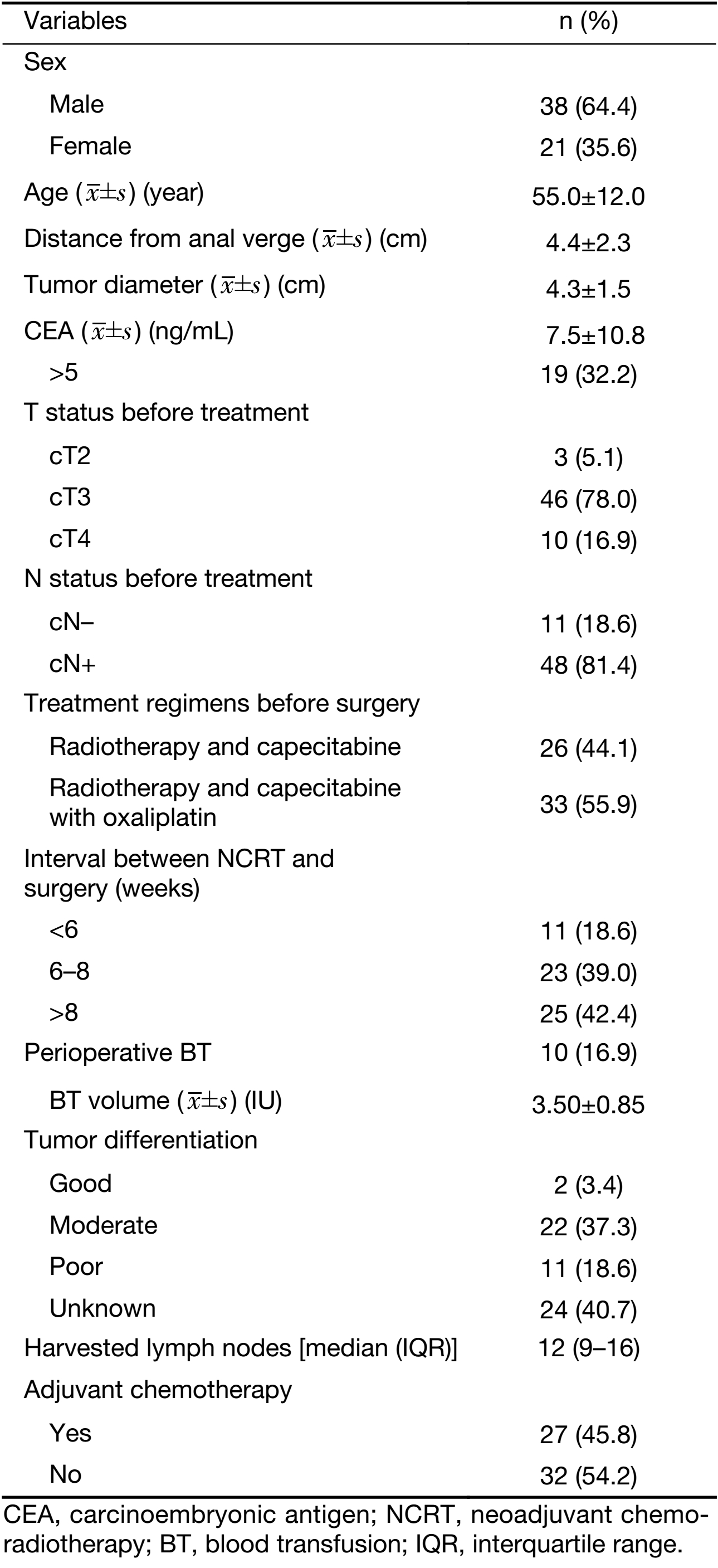
A total of 323 patients were reviewed. Upon review of the clinical records, ypT0 was achieved in 60 (18.6%) patients and one patient was excluded due to lack of follow-up. The vast majority of these remaining 59 patients enrolled were males (64.4%), their mean age was of 55.0 years, and tumors were mainly located in the low-rectum (mean distance from the anal verge 4.4 cm). The majority of the tumors were clinically staged as cT3 (78.0%) and cN+ (81.4%). Fifty patients (84.7%) received a total radiotherapy dose of 50 Gy in 25 fractions. Twenty-six patients (44.1%) were treated with radiotherapy and concurrent single-agent capecitabine, while thirty-three patients (55.9%) received radiotherapy and concurrent capecitabine with oxaliplatin. Surgery was performed at a median of 7.7 (IQR, 6.6–9.4) weeks after completion of NCRT. All patients underwent R0 resection with negative distal and circumferential margins. During the perioperative period, 10 patients (16.9%) received blood transfusion (BT; mean volume 3.5 IU). Twenty-seven (45.8%) patients received postoperative chemotherapy with regimens including: single agent capecitabine (n=5); capecitabine and oxaliplatin (XELOX) (n=21); 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX) (n=1). Thirty-two (54.2%) patients did not accept any adjuvant chemotherapy. The median total number of lymph nodes harvested was 12 (IQR, 9–16) and did not differ between the ypN0 and ypN+ patients (Table 1).
Full table
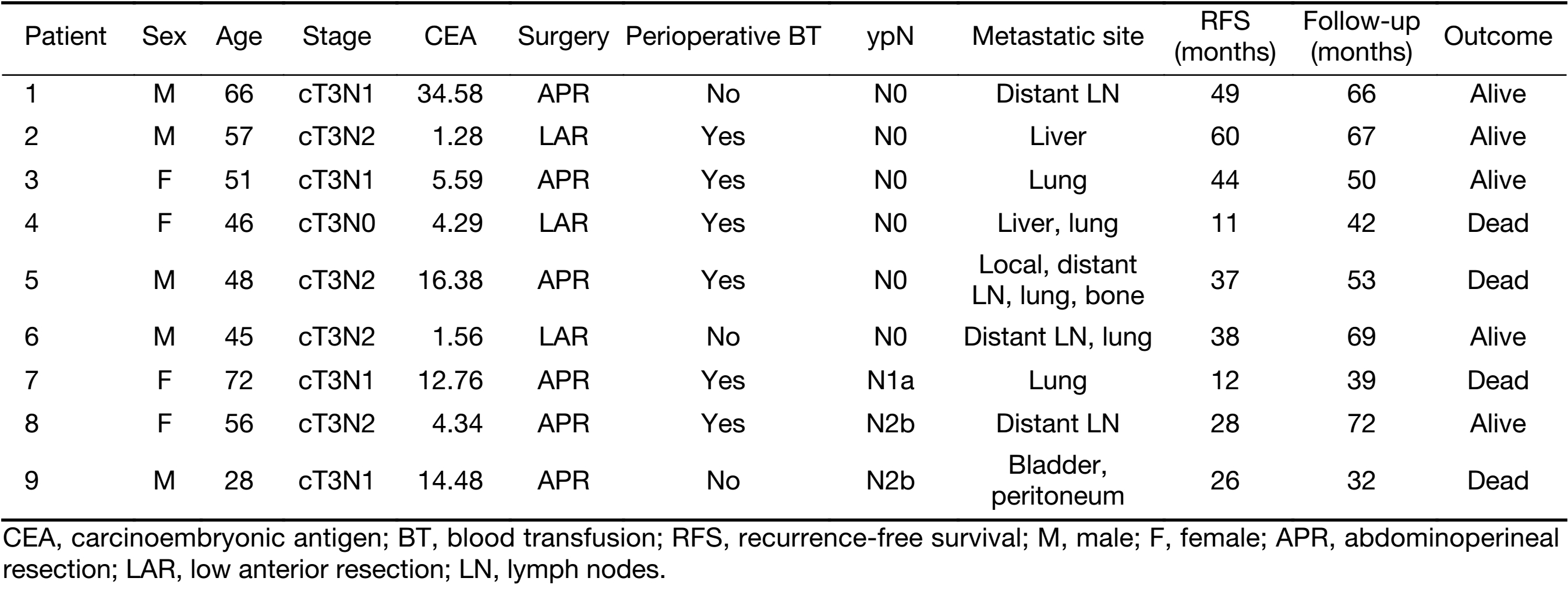
Pathological evaluation of specimens from the 59 patients showed that 51 patients (86.4%) had a complete pathological response to NCRT (ypT0N0), and eight patients (13.6%) had lymph node metastases in the mesorectum (ypT0N+). Of the eight patients with ypT0N+ disease, three patients had metastasis in only one lymph node, two patients had two metastatic lymph nodes, one patient had seven metastatic lymph nodes, one patient had 16 metastatic lymph nodes, and one patient showed one tumor deposit in the mesorectum.
Oncological outcomes
The median follow-up was 52 (IQR, 37–67; range, 29–136) months. Overall, tumor recurrence was observed in nine (15.3%) of the 59 patients, eight had distant recurrence, and one had both local and distant recurrences (Table 2). The mean interval from surgery to recurrence was 33.9 (x±s, 16.4; range, 11.0–60.0) months. Of the 59 patients, five patients (8.5%) died: four from recurrence and one from other causes. The mean interval from surgery to death was 42 (x±s, 7.6; range, 32.0–53.0) months.
Full table
Of the 51 patients with ypT0N0 disease, six (11.8%) developed distant recurrence, with one patients having both local and distant recurrences. Of the eight patients with ypT0N+ disease, three (37.5%) developed distant recurrence. Of the four patients who died of distant disease, two were classified as ypT0N0 and ypT0N+, respectively. The 5-year RFS rate was 82.7% in ypT0N0 patients and 62.5% in ypT0N+ patients (P=0.014). The 5-year OS rate was 90.9% for ypT0N0 and 70.0% for ypT0N+ (P=0.009).
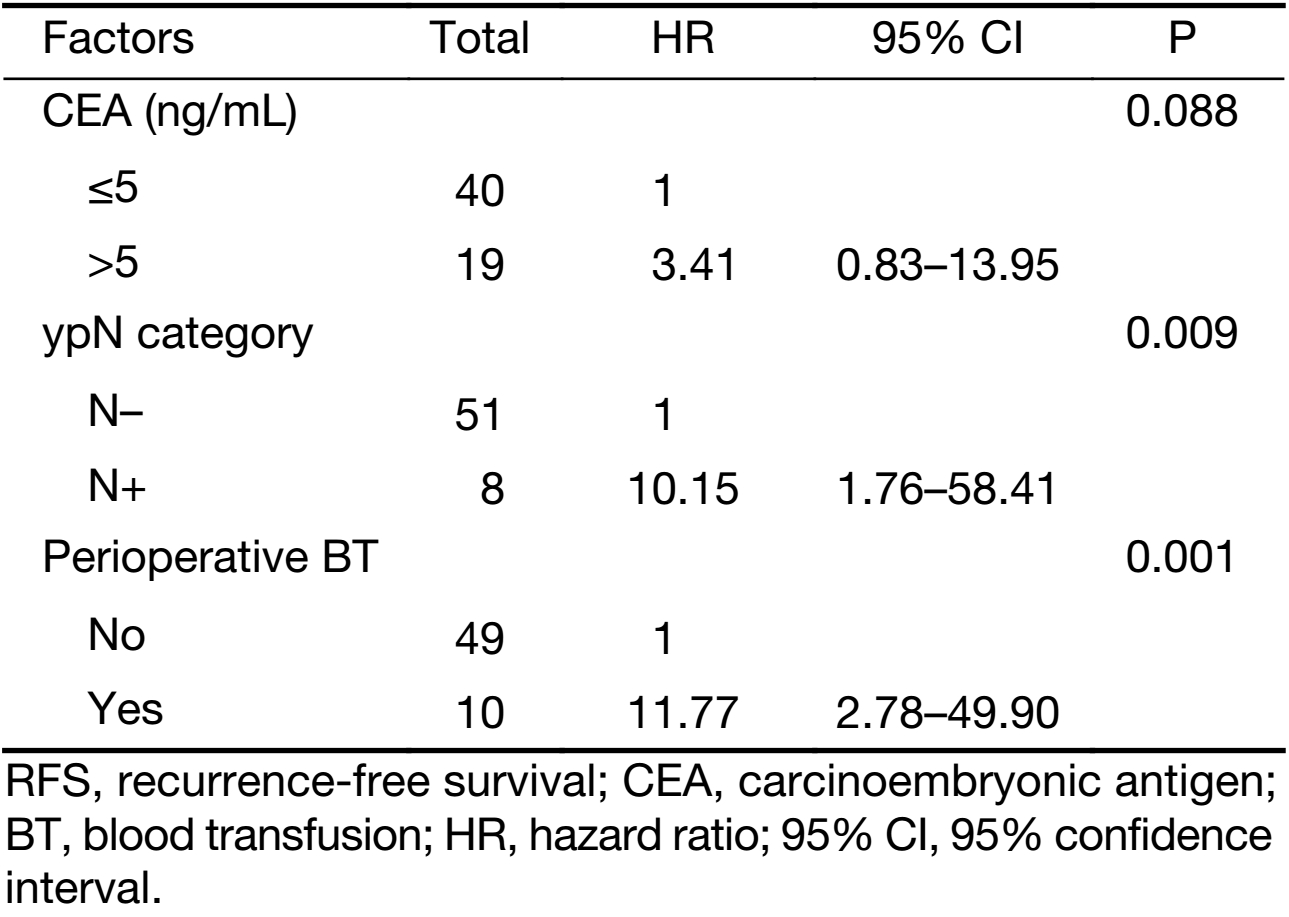
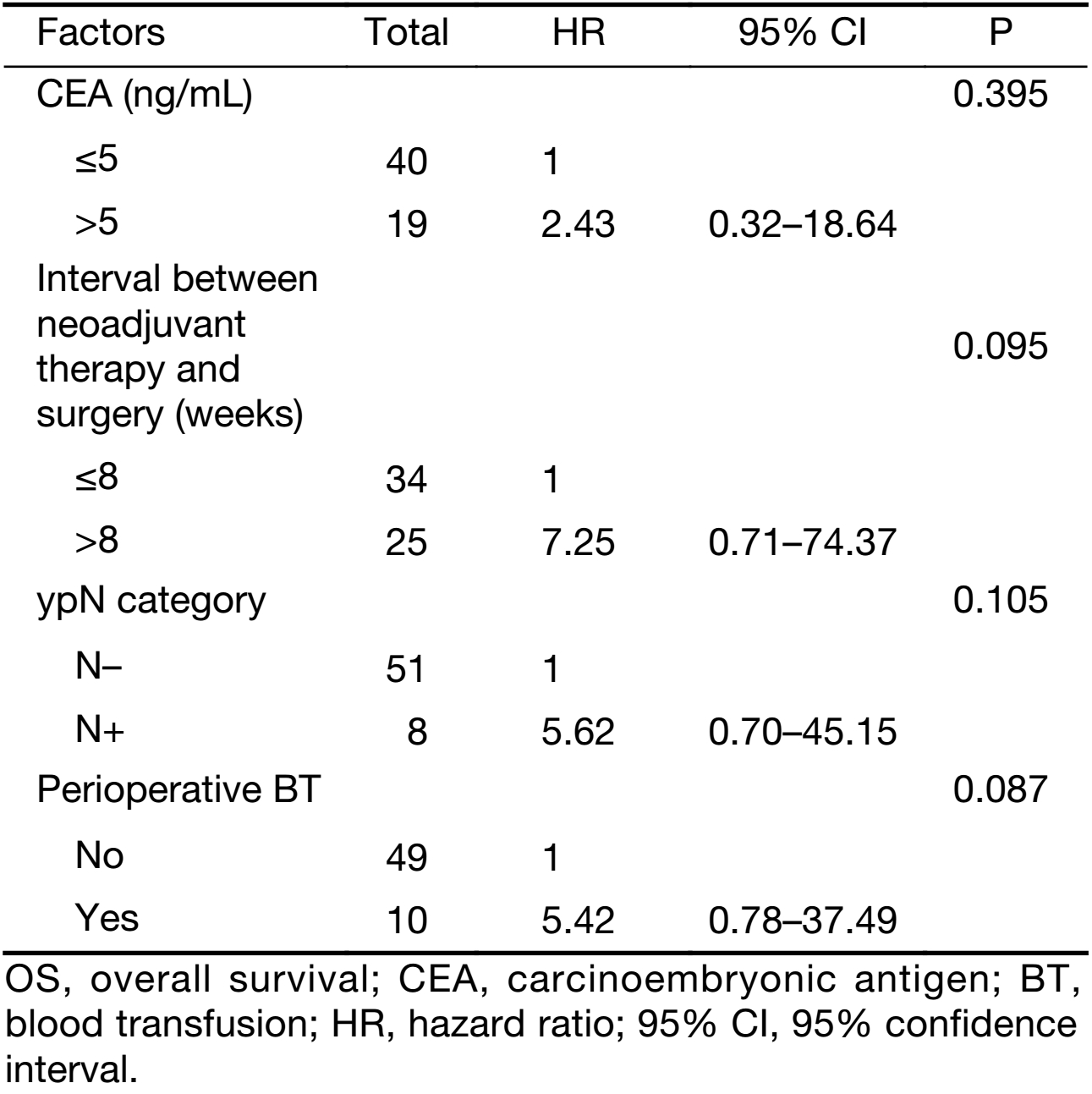
As shown in Table 3, the results of univariate analyses showed that RFS was significantly associated with ypN+ status (P=0.014) and perioperative BT (P<0.001). OS was significantly associated with interval between NCRT and surgery (P=0.038), ypN+ status (P=0.032), and perioperative BT (P=0.037). Multivariate analyses showed ypN+ status and perioperative BT to be independent prognostic factors correlated with decreased 5-year RFS; however, no factor was associated with OS (Tables 4 and 5).

Full table
Full table
Full table
Discussion
Over the past three decades, crucial improvements in the treatment of rectal cancer have been achieved with the introduction of NCRT and the standardization of the TME surgical technique (21,22). Currently, the guidelines of both the European Society of Medical Oncology and the National Comprehensive Cancer Network recommend performing NCRT in patients with LARC (2,3). This approach has several advantages, including down-sizing and down-staging of the primary tumor and a decreased local recurrence rate. The best outcome of NCRT is to obtain a pCR, which means complete eradication of all viable tumor cells from the rectal wall and mesorectum. Interestingly, NCRT leads to pCR rates varying from 10% to 27% (5-7), and we reported a pCR rate of 15.8% in the present study.
Currently, curative surgery with TME is still considered the standard surgical approach in patients with LARC after completion of NCRT. Although NCRT-induced inflammation, fibrosis and tissue edema might have adverse effects on the operation, several reports have indicated that NCRT in patients with LARC treated with radical surgery was not correlated with a higher incidence of postoperative complications, including anastomotic leakage (23,24). Moreover, radical surgery to obtain a final pCR is associated with favorable oncological outcomes. An Italian study analyzed 566 ypT0N0 patients from 36 centers and found that, after a median follow-up of 46.4 months, locoregional relapse, distant metastasis, 5-year DFS, OS, and cancer-specific survival rates were 1.6%, 8.9%, 85%, 90%, and 94%, respectively (5). A pooled analysis including 484 pCR patients with a median follow-up of 48 months found that patients with pCR after NCRT and surgery achieved favorable oncological outcomes compared with those with residual disease (DFS: 83.3% vs. 65.6%, P<0.0001) and the survival benefits of pCR on survival were not affected by clinical T or N status, administration of adjuvant chemotherapy, tumor location, or type of surgery (6). Another meta-analysis conducted by Zorcolo et al., analyzed the data for 1,913 patients from twelve studies and found that the 300 patients with pCR had better OS and DFS than those with partial or no response (OS: 92.9% vs. 73.4%, P=0.002; DFS: 86.9% vs. 63.9%, P=0.002) (7). The results of our study were similar to the above-noted studies, with 5-year RFS and OS rates of 82.7% and 90.9%, respectively, for ypT0N0 patients.
All of the studies mentioned above indicate that pCR translates to favorable oncological survival for patients. Based on this observation, and in an effort to preserve continence and long-term functional outcomes, avoid a permanent stoma, and reduce the morbidity and length of hospital stay associated with radical surgery, there has been a great interest in LE of the primary tumor or a watchful waiting strategy for patients who show a good clinical response to NCRT. In 2001, Kim et al. reported that 17 patients who underwent LE of their primary tumor after NCRT to achieve ypT0 disease did not experience recurrence, with a mean follow-up of 24 months (9). In 2004, Habr-Gama et al. indicated that omission of radical surgery with a watchful waiting policy might be workable in selected patients who achieved a clinical complete response (cCR) to NCRT (10). Increasing evidence suggests that these methods are feasible in less advanced tumors (11-13).
However, when considering LE or a watchful waiting policy, these questions should be taken into account. Firstly, how should appropriate imaging technology be selected to characterize cCR or a complete response of the primary tumor? In 2013, Guillem et al. found that neither PET nor CT scans had adequate predictive ability to identify a pCR from an incomplete response (25). Even using combined tools (EUS and MRI), the sensitivity to select a pCR or sustained cCR patients was just 18.2% (26). Secondly, how should regrowths or recurrences be managed after NCRT and LE of the primary tumor or a watchful waiting policy? In review of the medical literature, the incidence of patients with rectal cancer who develop local regrowths after a watchful waiting strategy following NCRT ranges from 15% to 60% (12,13,27-30). Although most of these patients receive salvage resection, it is unclear whether NCRT-induced fibrosis limits the feasibility of salvage surgery, and whether salvage surgery is associated with increased postoperative complications. Moreover, the long-term outcomes of salvage surgery are also unclear. Recently, Perez et al. suggested that salvage resection for local recurrences after NCRT and transanal endoscopic microsurgery (TEM) was correlated with a high rate of R1 resection with positive circumferential margin in 87% of patients. Even if salvage surgery was carried out, the 2-year local re-RFS was just 60% (31). Another issue that should not be ignored is the status of mesorectal lymph nodes, which may be an important prognostic factor for local and distant recurrences. Several studies have shown that nodal metastasis in the mesorectum is recognized to be one of the most independent risk factors for recurrence (32,33). Although patients with lower ypT classifications following NCRT were less likely to have ypN+ status, the incidence of metastatic lymph node involvement among patients with ypT0 status after NCRT and surgery in prior reports varies from 6.6% to 16% (15-20).
In our study, 13.6% of patients with ypT0 rectal cancer after NCRT and curative surgery were ypN+ disease, consistently with prior results, and distant recurrence occurred more frequently in the ypN+ than the ypN0 patients. Recently, an Italian study of 261 patients who presented a complete or nearly complete response of primary tumor from 13 centers showed that nodal positivity accounted for 8.7% of patients, and multivariate analyses identified nodal metastasis in the mesorectum as the only risk factor independently associated with a decreased OS (20). In accordance with this, Loftås et al. analyzed 161 ypT0 patients at a national level, revealing that survival was significantly better in ypT0N0 patients compared with ypT0N+ patients, and concluding that the survival benefits of primary tumor complete response were contingent upon ypN status (19). Similarly, according to the Korean Radiation Oncology Group study, residual nodal disease in ypT0 patients was associated with decreased DFS and OS (16). Another smaller cohort study of 91 ypT0 patients conducted by Jang et al. also suggested that ypN+ status was a significant prognostic factor correlated with distant metastases (17).
In summary, long-term outcomes in patients with ypT0N0 rectal cancer are excellent. However, patients with residual disease in the mesorectum even after complete regression of primary tumor have a poorer outcome. Thus, caution should be taken when using LE of primary tumor or a watchful waiting management approach for these patients.
For many years, BT has been an indispensable part of the standard treatment used alongside the surgery in cancer patients. Although BT has been recognized to improve oxygen delivery and tissue perfusion, it is also associated with a poorer outcome in cancer patients, especially in colorectal cancer. According to the results of the American College of Surgeons National Surgical Quality Improvement Program, 14.07% of patients received BT, and BT was correlated with worse short-term outcomes, for example, increased mortality and morbidity, longer hospital stay, and higher incidence of pulmonary and wound infection (34). Several studies also suggested that for patients with colorectal liver metastases undergoing liver resection, 5-year OS and RFS were worse in patients who received blood (35,36). However, the possible mechanism by which BT is associated with worse outcomes is unclear. Some deem that the immunomodulatory effect of BT might induce decreased macrophage function, lower CD4/CD8 rations, and reduced natural killer cells function which may contribute to a reduced immune surveillance, thereby stimulating tumor growth, tethering, and dissemination (37,38). On the contrary, others have suggested that worse survival after rectal surgery in patients receiving BT was due to the clinical circumstances requiring transfusion, not because of the BT itself (39). In the present study, 16.9% of patients received BT and BT was significantly associated with reduced RFS and OS. After adjustment for prognostic factors, BT was independently correlated with decreased RFS [hazard ratio (HR): 11.77; 95% confidence interval (95% CI): 2.78–49.90; P=0.001]. Although most patients with LARC after NCRT have poor performance status, it is unclear whether BT is more likely to affect immune defense, or to stimulate tumor growth, tethering, and dissemination in these patients. Future studies are warranted to illuminate this point.
This study had several limitations. First, the sample size for patients in the ypN0 and ypN+ groups was small which may limit the statistical analysis. Second, being a retrospective study and based on a single-center setting, it might be susceptible to selection bias. Besides, due to pCR of primary tumor after NCRT and surgery, tumor differentiation was not available for 40.7% of patients, although pretreatment pathological examination was performed for all patients before NCRT, which might decrease the statistical power owing to the high percentage of unknown data. Although we documented perioperative BT as an independent risk factor for recurrence, we did not further identify predictive factors independently associated with the necessity for transfusion.
Conclusions
Long-term outcomes in patients with ypT0N0 rectal cancer are excellent; however, positive lymph nodes or tumor deposits can still be found in 13.6% of patients who show complete pathological response in the rectal wall and are independently associated with decreased 5-year RFS. Additionally, perioperative BT is also one variable independently correlated with a worse RFS, so effective ways to rationalize blood utilization in rectal cancer surgery following NCRT are warranted to minimize the adverse effect on survival.
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2017YFC0908203) and CAMS Initiative for Innovative Medicine (No. CAMS-I2M-003).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1–10. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.01.01
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28(Suppl 4):iv22–40. [PubMed] DOI:10.1093/annonc/mdx224
- National Comprehensive Cancer Network (2017) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Rectal cancer (Version 1. 2017). Available online: http://www.nccnorg/professionals/physician_gls/pdf/rectalpdf
- Stipa F, Chessin DB, Shia J, et al. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol 2006;13:1047–53. [PubMed] DOI:10.1245/ASO.2006.03.053
- Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99–107. [PubMed] DOI:10.1016/j.ijrobp.2007.12.019
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. [PubMed] DOI:10.1016/S1470-2045(10)70172-8
- Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 2012;19:2822–32. [PubMed] DOI:10.1245/s10434-011-2209-y
- Wasmuth HH, Rekstad LC, Tranø G. The outcome and the frequency of pathological complete response after neoadjuvant radiotherapy in curative resections for advanced rectal cancer: a population-based study. Colorectal Dis 2016;18:67–72. [PubMed] DOI:10.1111/codi.13072
- Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg 2001;234:352–8. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711–7. [PubMed] DOI:10.1097/01.sla.0000141194.27992.32
- Hallam S, Messenger DE, Thomas MG. A systematic review of local excision after neoadjuvant therapy for rectal cancer: Are ypt0 tumors the limit? Dis Colon Rectum 2016;59:984–97. [PubMed] DOI:10.1097/DCR.0000000000000613
- Martens MH, Maas M, Heijnen LA, et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst 2016;108:pii:djw171. [PubMed] DOI:10.1093/jnci/djw171
- Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174–83. [PubMed] DOI:10.1016/S1470-2045(15)00467-2
- Cotte E, Passot G, Decullier E, et al. Pathologic response, when increased by longer interval, is a marker but not the cause of good prognosis in rectal cancer: 17-year follow-up of the Lyon r90-01 randomized trial. Int J Radiat Oncol Biol Phys 2016;94:544–53. [PubMed] DOI:10.1016/j.ijrobp.2015.10.061
- Park IJ, You YN, Skibber JM, et al. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum 2013;56:135–41. [PubMed] DOI:10.1097/DCR.0b013e318278ff8a
- Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010;252:998–1004. [PubMed] DOI:10.1097/SLA.0b013e3181f3f1b1
- Jang TY, Yu CS, Yoon YS, et al. Oncologic outcome after preoperative chemoradiotherapy in patients with pathologic T0 (ypT0) rectal cancer. Dis Colon Rectum 2012;55:1024–31. [PubMed] DOI:10.1097/DCR.0b013e3182644334
- Vallam KC, Engineer R, Desouza A, et al. High nodal positivity rates even in good clinical responders after chemoradiation of rectal cancer: is organ preservation feasible? Colorectal Dis 2016;18:976–82. [PubMed] DOI:10.1111/codi.13114
- Loftås P, Arbman G, Fomichov V, et al. Nodal involvement in luminal complete response after neoadjuvant treatment for rectal cancer. Eur J Surg Oncol 2016;42:801–7. [PubMed] DOI:10.1016/j.ejso.2016.03.013
- Lorenzon L, Parini D, Rega D, et al. Long-term outcomes in ypT0 rectal cancers: An international multi-centric investigation on behalf of Italian Society of Surgical Oncology Young Board (YSICO). Eur J Surg Oncol 2017;43:1472–80. [PubMed] DOI:10.1016/j.ejso.2017.04.017
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184–90. [PubMed] DOI:10.1016/S1470-2045(13)70599-0
- Kapiteijn E, Putter H, van de Velde CJ, et al. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142–9. [PubMed] DOI:10.1046/j.1365-2168.2002.02196.x
- Valenti V, Hernandez-Lizoain JL, Baixauli J, et al. Analysis of early postoperative morbidity among patients with rectal cancer treated with and without neoadjuvant chemoradiotherapy. Ann Surg 2007;14:1744–51. [PubMed] DOI:10.1245/s10434-006-9338-8
- Martel G, Al-Suhaibani Y, Moloo H, et al. Neoadjuvant therapy and anastomotic leak after tumor-specific mesorectal excision for rectal cancer. Dis Colon Rectum 2008;51:1195–201. [PubMed] DOI:10.1007/s10350-008-9368-3
- Guillem JG, Ruby JA, Leibold T, et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg 2013;258:289–95. [PubMed] DOI:10.1097/SLA.0b013e318277b625
- Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, et al. Pathologic complete response in rectal cancer: can we detect it? Lessons learned from a proposed randomized trial of watch-and-wait treatment of rectal cancer. Dis Colon Rectum 2016;59:255–63. [PubMed] DOI:10.1097/DCR.0000000000000558
- Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol 2010;49:378–81. [PubMed] DOI:10.3109/02841860903483692
- Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256:965–72. [PubMed] DOI:10.1097/SLA.0b013e3182759f1c
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015;16:919–27. [PubMed] DOI:10.1016/S1470-2045(15)00120-5
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822–8. [PubMed] DOI:10.1016/j.ijrobp.2013.12.012
- Perez RO, Habr-Gama A, São Julião GP, et al. Transanal endoscopic microsurgery (TEM) following neoadjuvant chemoradiation for rectal cancer: outcomes of salvage resection for local recurrence. Ann Surg Oncol 2016;23:1143–8. [PubMed] DOI:10.1245/s10434-015-4977-2
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688–96. [PubMed] DOI:10.1200/JCO.2005.02.1329
- Kim TH, Chang HJ, Kim DY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys 2012;77:1158–65. [PubMed] DOI:10.1016/j.ijrobp.2009.06.019
- Halabi WJ, Jafari MD, Nguyen VQ, et al. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg 2013;206:1024–32. [PubMed] DOI:10.1016/j.amjsurg.2013.10.001
- Schiergens TS, Rentsch M, Kasparek MS, et al. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum 2015;58:74–82. [PubMed] DOI:10.1097/DCR.0000000000000233
- Hallet J, Tsang M, Cheng ES, et al. The impact of perioperative red blood cell transfusions on long-term outcomes after hepatectomy for colorectal liver metastases. Ann Surg Oncol 2015;22:4038–45. [PubMed] DOI:10.1245/s10434-015-4477-4
- Blajchman MA. Immunomodulation and blood transfusion. Am J Ther 2002;9:389–95. [PubMed]
- Kirkley SA. Proposed mechanisms of transfusion-induced immunomodulation. Clin Diagn Lab Immunol 1999;6:652–7. [PubMed]
- Warschkow R, Güller U, Köberle D, et al. Perioperative blood transfusions do not impact overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg 2014;259:131–8. [PubMed] DOI:10.1097/SLA.0b013e318287ab4d
