Presence of circulating tumor cells is associated with metabolic-related variables in postoperative patients with early-stage breast cancer
Introduction
Circulating tumor cells (CTCs) that detach from the primary tumor and circulate in the peripheral blood have the potential to form distant metastases. It has been well-documented that the presence of CTCs in early breast cancer is associated with future development of recurrent metastasis (1). Accumulated studies have established the critical role for CTCs in predicting unfavorable survival rates for metastatic breast cancer (MBC) patients (2-6). Consistent with these findings, a previous multi-center clinical trial in China (CBCSG004, China Breast Cancer Clinical Study Group) by our group not only confirmed that the presence of ≥5 CTCs before and after a new line of systemic therapy was significantly associated with shorter progression-free survival (PFS) [hazard ratio: 1.93; 95% confidence interval (95% CI): 1.39–2.69; P<0.001] and overall survival (OS) (hazard ratio: 3.76; 95% CI: 2.35–6.01; P<0.001) but also found that the prognostic value of CTCs varied in different disease subtypes of MBC in Chinese patients (5).
In keeping with these findings, the prognostic value of CTCs in patients with non-MBC, as assessed at the time of primary diagnosis, has been shown convincingly in a recent large pooled analysis involving 3,173 patients (6). This analysis additionally demonstrated that the presence of CTCs was positively associated with a larger tumor size, intensified lymph node involvement and unfavorable histological grade. The SUCCESS trial (EUDRA-CT No. 2005-000490-21) first reported the time-related prognostic associations of CTCs in non-MBC patients. The prevalence of CTCs was evaluated after two follow-up periods; CTCs were detected in 18.6% and 8.5% of patients at 2 and 5 years, respectively (7). At the 2017 San Antonio Breast Cancer Symposium (SABCS), Sparano et al. presented evidence that the presence of CTCs in peripheral blood, even 5 years after primary diagnosis, was associated with an 18.3-fold increased risk of late recurrence in hormone receptor (HR)-positive breast cancer patients (8). This finding underlines the clinical validity of CTC as a prognostic biomarker for late recurrence in HR-positive breast cancer. More importantly, the rate of positive CTC results was 4.8% at five years after diagnosis, and the presence of CTC was not associated with any specific patient characteristics in this study. This finding raised the possibility of tumor dormancy in relapse-free patients – detectable CTCs decreased gradually, and the correlations between CTCs and pathological characteristics weakened over time.
Although the prognostic value of CTCs in breast cancer has been well-established, the risk factors associated with the presence of CTCs have not been well-evaluated, especially in long-term follow-up. Diseases related to metabolic dysfunction, such as obesity and hyperglycemia, have recently been considered “emergent hallmarks” of cancer, representing the novel notion that tumor cells could reprogram metabolism to adapt to the active neoplastic proliferative state (9). Remarkably, meta-analyses including large numbers of patients have provided evidence that obesity increased the risk of recurrence and mortality rates by approximately 35%–40% (10). Nevertheless, the potential relevance that underlies the relationship between metabolic-related variables and CTCs has not been explored to date. Herein, we investigated the correlations of CTCs with clinicopathological characteristics and various metabolic-related variables from a unique perspective to determine any potential relationship between patient metabolism and CTCs as a surrogate for disease burden.
Materials and methods
Participants
In this retrospective study, we recruited 264 patients with non-MBC who had completed breast cancer surgery treatment at Guangdong General Hospital from January 2009 to December 2015. Eligible patients were defined as women with histologically confirmed, operable, stage I–III invasive breast cancer without clinical evidence of metastasis (stages pT1–T3, pN0–N3, M0). Patients who were diagnosed with other malignancies (such as ovarian cancer or endometrial cancer) were ineligible, as were those who were not compliant with treatment regimens. Informed consent was obtained from all patients before blood collection, and the study was approved by the Institutional Review Board of Guangdong General Hospital [IRB NO. GDREC2012113H(R1)].
Patient and clinicopathological characteristics were collected for all participants. The tumor, node, metastasis (TNM) staging system stage at primary diagnosis was classified according to the revised American Joint Commission on Cancer (AJCC) 7th edition guidelines (11). Histological grading of the primary tumor was assessed using the Nottingham system. Tumors in which immunohistochemical nuclear staining for estrogen receptor (ER), progesterone receptor (PR), or both yielded ≥10% were classified as HR-positive. Human epidermal growth factor receptor 2 (HER2) positivity was defined if strong (3+) immunohistochemical membranous staining was present or, in the case of moderate (2+) membranous staining, if an additional fluorescence in situ hybridization (FISH) test yielded a positive result. The molecular classification of the enrolled patients was confirmed according to the 2015 St. Gallen expert consensus (12).
Sample collection and CTC detection
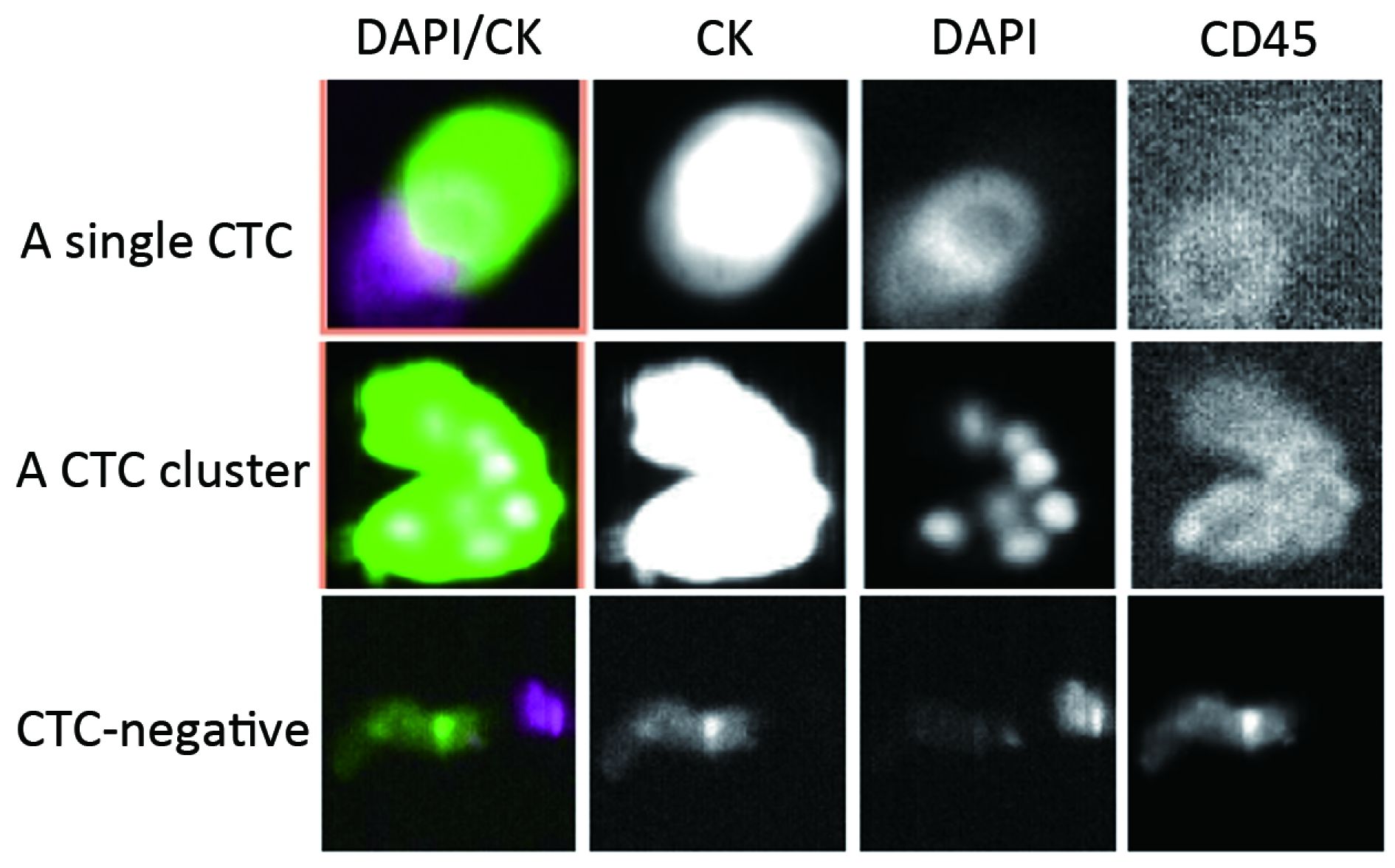
For each enrolled patient, 7.5 mL of peripheral blood was drawn into CellSave tubes (Janssen Diagnostics, LLC, Raritan, NJ, USA), which were shipped to the laboratory and analyzed within 72 h. The median time between the sample and surgery was 19.0 months [interquartile range (IQR), 7.8–33.0]. Before CTC analysis, the samples were centrifuged for 10 min at 800× g separate the solid blood components from the plasma then dilution buffer was added to the blood components. The prepared samples were processed using the CellTracks Autoprep system, which was loaded with the CellSearch kit (Menarini Silicon Biosystems Inc., PA, USA) within 1 h. After the epithelial-specific marker (EpCAM) antibody was immunomagnetized and enriched, immunofluorescence staining with an anti-keratin antibody (CK8,18,19-phycoerythrin), an anti-CD45 antibody (CD45-allophycocyanin) and an anti-nuclear dye (DAPI, nucleic acid dye 4’,6-diamidino-2-phenylindole) was performed to detect intact tumor cells.
CTCs were identified and enumerated on a CellTracks Analyzer II. CTCs were defined as nucleated cells with round to oval morphology that were DAPI-positive, CD45-negative, and CK-positive. All positive specimens were examined by two independent investigators. Considering that the recruited patients were diagnosed with non-MBC, they were divided into the CTC-positive (CTC≥1/7.5 mL blood samples) and CTC-negative (CTC=0/7.5 mL blood samples) groups according to the CTC results.
In addition, fasting blood was collected for assessment of such metabolic variables as the levels of glucose, lipoprotein A, triglycerides, free fatty acid (FFA), total cholesterol (TCH), low-density lipoprotein (LDL), apolipoprotein A1, apolipoprotein B100, high-density lipoprotein (HDL) and uric acid. We also collected information about any history of diabetes, hypoglycemic agent treatment and tested glycated hemoglobin (HbA1c) level in patients with hyperglycemia. The levels were compared with the normal values, and the details are as follows: glucose (3.89–6.11 mmol/L), lipoprotein A (0–300 mg/L), triglycerides (0.56–1.70 mmol/L), FFA (0.10–0.45 mmol/L), TCH (3.1–5.7 mmol/L), LDL (2.7–4.1 mmol/L), apolipoprotein A1 (1.2–1.6 g/L), apolipoprotein B100 (0.80–1.05 g/L), HDL (1.29–1.55 mmol/L), uric acid (89–357 mmol/L) and HbA1c (<6.1%).
Statistical analyses
The demographic and clinical characteristics were analyzed using the Pearson Chi-square test (categorical variables) and the t-test (continuous variables). Comparisons for the non-normally distributed metric variable were conducted using the Mann-Whitney U test. The results for the metabolic factors were divided into two groups according to their normal values before they were analyzed by the Pearson Chi-square test. The Fisher exact test was applied when the theoretical frequency in 1/5 of the table was less than five or when any one of the theoretical frequencies was less than one. To further analyze the independent predictors of CTCs, a multivariate logistic regression analysis with a stepwise selection process was used to evaluate the relevant clinical features of CTCs. In this analysis, the continuous variables included were age, HDL and blood glucose, and the categorical variables included were menstrual state, pathological type, TNM stage, HER2 status, HR/HER2 status, systemic therapy, history of diabetes and hypoglycemic agent treatment. It was worth mentioning that we included the history of diabetes and hypoglycemic agent treatment in the regression analysis to exclude these confounders of blood glucose. A stringency level (P-value) of 0.05 was considered to be significant. All data were analyzed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA).
Results
Correlations between CTCs and clinicopathological features
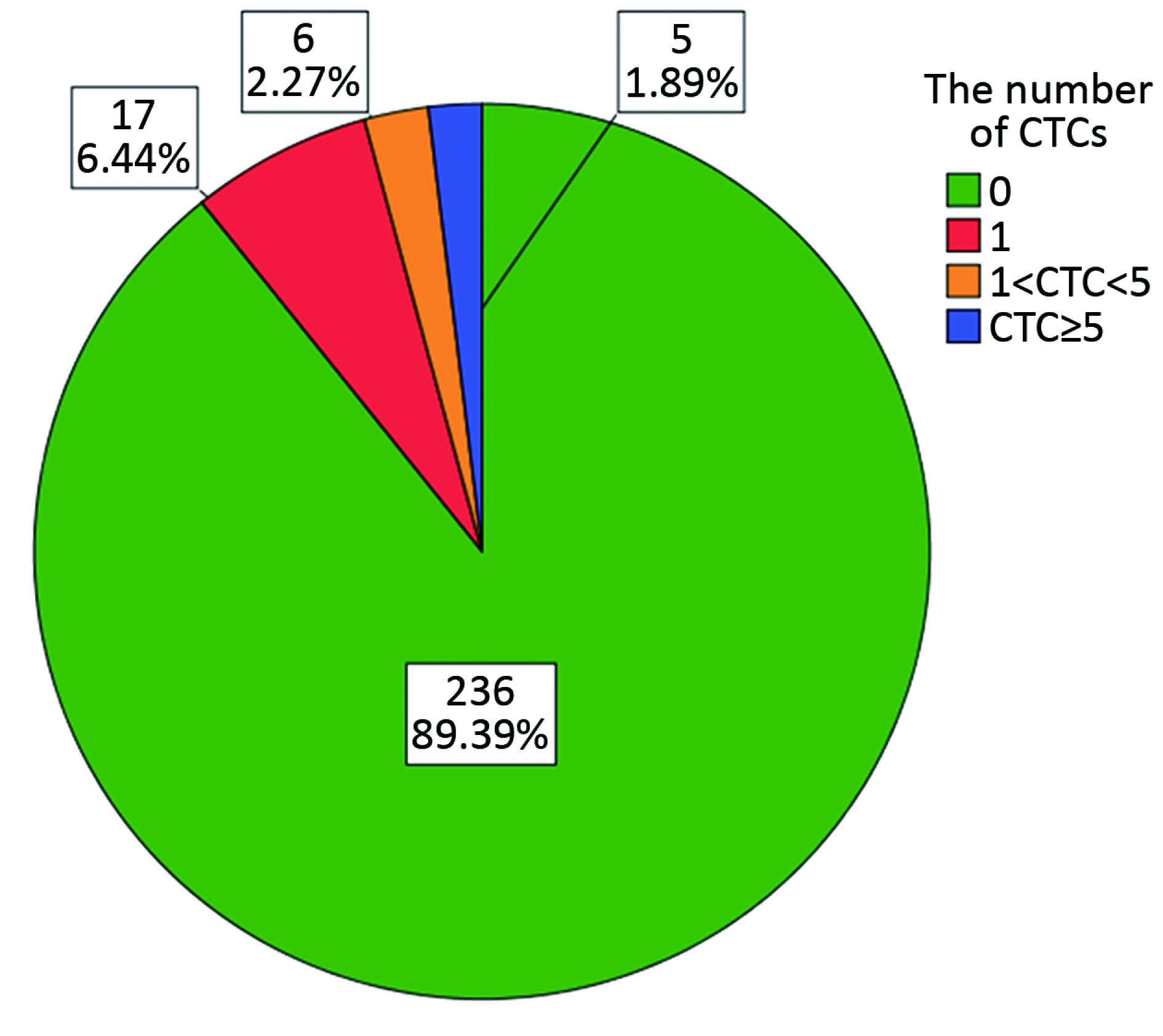
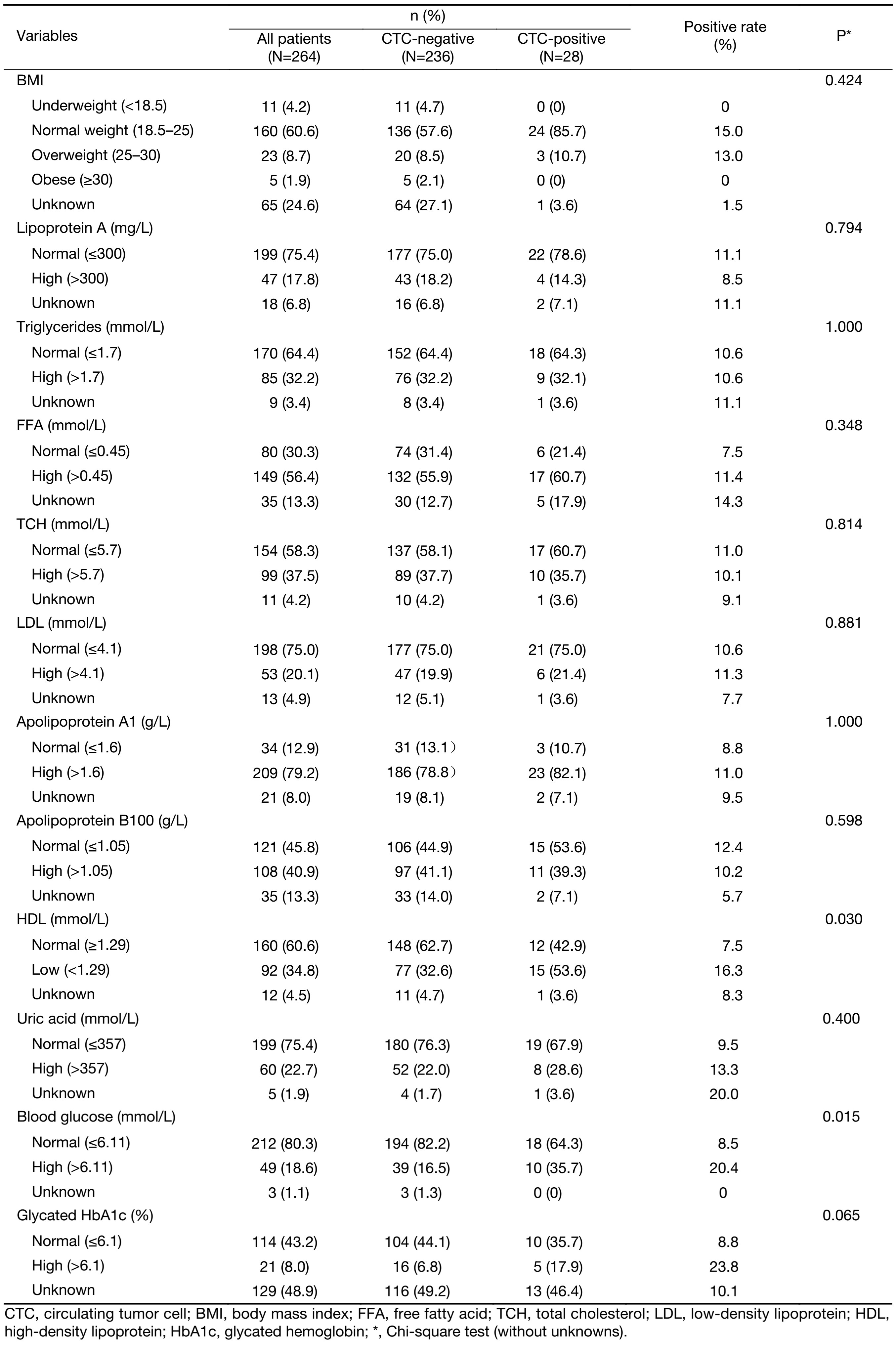
Peripheral blood samples from 264 patients with non-MBC who had completed breast cancer primary surgical treatment were collected. The mean age of the patients was 48.6±11.3 (range: 26–84) years. The prevalence and numbers of CTCs were assessed using the CellSearch System at a median time of 19.0 months (IQR, 7.8–33.0) after surgery. The demographic and clinical characteristics of the participants according to the CTC results are shown in Table 1. CTCs were detected in 28 patients (10.6%); 23 patients (82.1%) exhibited counts of 1 to 4 CTCs and 5 patients (17.9%) had 5 or more CTCs (Figure 1, 2). The number of CTCs observed ranged from 0 to 108 (median 1, geometric mean 2.23). Consistent with the results reported by Sparano in 2017 SABCS, the presence of CTCs was not associated with age, tumor size, ER and/or PR+, lymph node status, or histological grade (Table 1). However, the positive rate of CTCs in patients with infiltrating ductal carcinoma (IDC) was lower than that in patients with other pathological types (9.0% vs. 11.1%, 28.6%, P=0.020) (Table 1).

Full table
Correlations between CTCs and metabolic factors
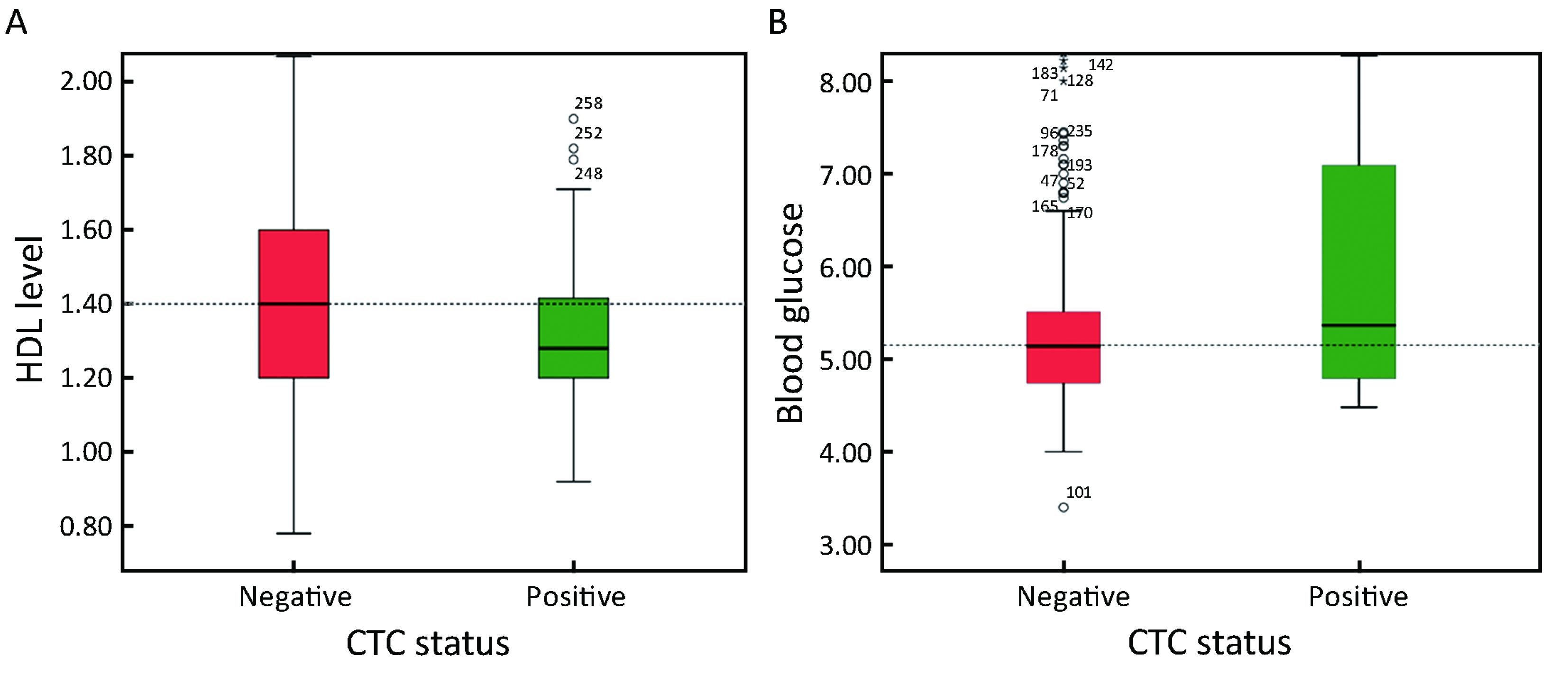
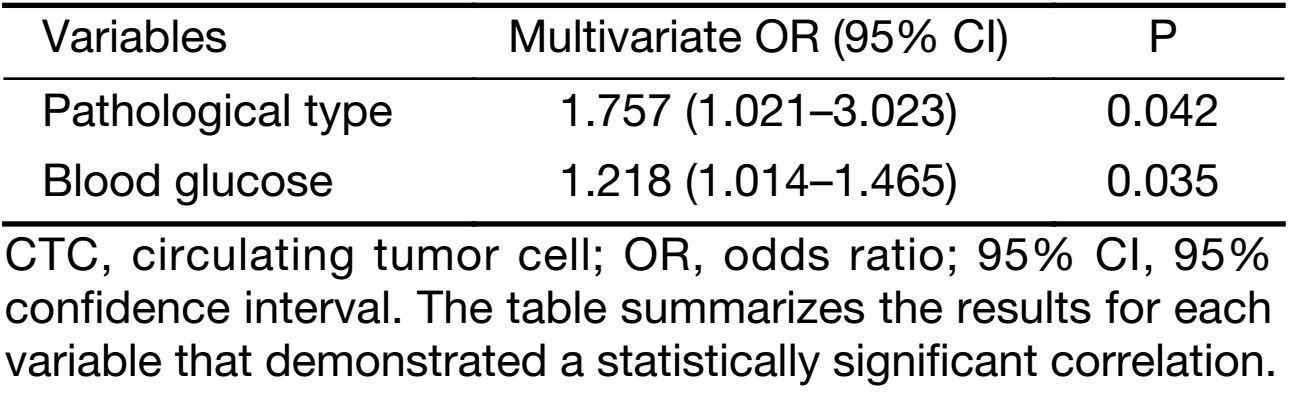
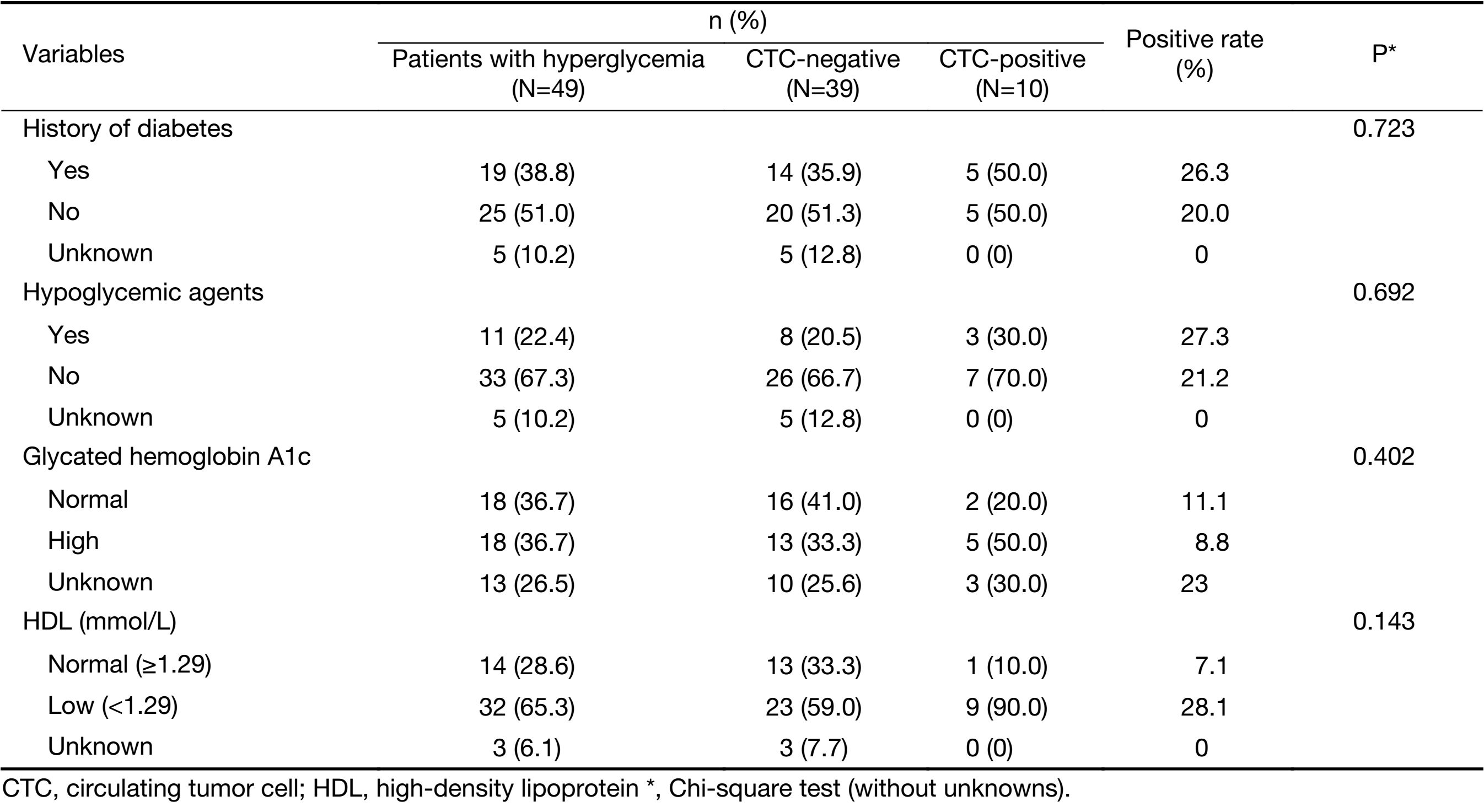
In this study, we further investigated the relationship between CTCs and metabolic factors. We found that the presence of CTCs was positively correlated with an elevated blood glucose (20.4% CTCs with elevated glucose versus 8.5% CTCs with normal glucose levels, P=0.015) and was negatively correlated with a normal level of HDL (16.3% CTCs with a low level versus 7.5% CTCs with a normal level; P=0.030) (Table 2, Figure 3). Moreover, HbA1c level was positively associated with CTC with a correlation level that neared significance (P=0.065). No associations were observed for body mass index (BMI) (P=0.424), triglycerides (P=1.000), TCH (P=0.814), LDL (P=0.881) and other metabolic related variables (Table 2). Based on the results of the multivariate analysis, pathological type [odds ratio (OR): 1.757, 95% CI: 1.021–3.023; P=0.042] and blood glucose level (OR: 1.218, 95% CI: 1.014–1.465; P=0.035) were found to be independent predictors of CTCs during the follow-up of Chinese patients with non-MBC (Table 3). Among the 49 cases of patients with hyperglycemia, 65.3% of the patients with (32 of 49) hyperglycemia had lower HDL level. However, there was no statistical significance of the positive rate of CTC between the low and normal HDL groups (28.1% vs. 7.1%, P=0.143). The CTC positive rate was not significantly different between the high and normal HbA1c group (Table 4).
Full table
Full table
Full table
Prediction of survival in the postoperative follow-up
Due to the limited follow-up period, two patients with detectable CTCs were diagnosed with metastasis via imaging examinations after CTC collection. One of these patients had stage III disease and 66 CTCs; the CT scan showed a large amount of left pleural effusion and pericardial effusion, both of which indicate pleural mediastinal metastases. The other patient had stage IIa disease and 9 CTCs and was diagnosed with liver metastasis according to the imaging examination. No recurrence or metastasis was detected among the 236 patients without detectable CTCs.
Discussion
CTCs were identified in 28 (10.6%) of 264 eligible patients in this cohort patients. The presence of CTCs was positively correlated with an elevated blood glucose (P=0.015) and negatively correlated with HDL (P=0.030). The multivariate analyses demonstrated that blood glucose level and pathological type were independent predictors of CTCs in the peripheral blood. This report describes the China-based study to explore the related predictors of CTCs via an analysis of the correlations of CTCs with clinicopathological characteristics and metabolic-related variables in the postoperative follow-up of non-MBC patients.
As previously stated, at the latest SABCS Sparano et al. presented a notable result of a prospective clinical trial that the presence of CTC was associated with an 18.3-fold increased risk of breast cancer recurrence in patients with HR-positive disease. This finding powerfully demonstrated the clinical validity of the CTC assay as a prognostic biomarker for late recurrence (8). In Sparano’s study, CTCs were measured in blood samples from 546 patients between 4.5 and 7.5 years after the initial diagnosis of HR-positive stage II–III breast cancer using the CellSearch system – 4.8% had a positive CTC result. Compared to the Sparano’s study, CTCs were assessed in 264 patients with early breast cancer using the CellSearch System at a median time of 19.0 months after surgery in our study; the positive rate of CTCs was 10.6%. Additionally, there were no associations between CTC and HR status in our study; this is in line with the results from Sparano’s study (Table 4) (8). As the results of the SUCCESS-A trial demonstrated, CTCs at 2 and 5 years were detected in 18.6% and 8.5% of patients respectively, implying that the positive rate for CTCs decreased gradually over time (7). We believe the divergence in the positive rate of CTCs between the two studies is likely attributed to the different time intervals between CTC detection and initial diagnosis.
Several studies have reported that metabolic dysfunctions such as obesity and hyperglycemia have a detrimental effect on the outcomes of patients with early breast cancer (13,14). A BMI≥30 kg/m2 is defined as the threshold of poorer survival outcomes in both pre- and post-menopausal women with breast cancer (15). However, the interactions between metabolic dysfunctions and breast cancer are complex, and there are few studies that identify the potential relationship between metabolic variables and CTCs in breast cancer. In this study, we observed that the presence of CTCs was not associated with BMI (Table 2). This conclusion was in agreement with recently published work that investigated the relationship between CTCs and BMI, as BMI was not independently associated with the presence of CTCs or positive pathological complete response (pCR) rates in inflammatory breast cancer (16). Compared with the previous studies that focused on the analysis of anthropometric measurements (height, weight, BMI), our study enrolled patients with non-MBC and investigated the association between CTCs and metabolic-associated laboratory indicators, including blood glucose, triglycerides, TCH, LDL and HDL. The data demonstrated that none of these indicators was associated with CTCs, except for blood glucose and HDL level. Moreover, HbA1c was positively associated with CTC in correlations that neared significance (P=0.065). In the 2015 study by Huang et al., the diabetic HDL in patients with diabetes has been shown to have the potential of promoting breast cancer metastasis by facilitating adherence of CTCs to the endothelium, a pivotal initial point in the metastasis cascade (17). Modifications via glycation and peroxidation may lead to the derangement of HDL metabolism and variations in the structure and morphology of HDL in pathological conditions. This abnormal and dysfunctional HDL has impaired vasoprotective capacities and may even become pro-inflammatory (18,19).
In this study, we further collected the medical history of diabetes and examined the HDL and HbA1c status in 49 cases where patients demonstrated hyperglycemia. As our results showed, 65.3% of the patients (32 of 49) with hyperglycemia had lower HDL levels; this finding echoed the results in Huang’s research in that the breast cancer patients with comorbid diabetes had lower levels of HDL (1.29±0.32 vs. 0.91±0.37, P<0.01) (17). However, there was no statistical significance observed between a positive rate of CTC between the low and normal HDL groups (28.1% vs. 7.1%, P=0.14). This result merits further investigation with a larger data set, and as we have more patients with hyperglycemia in each HDL class available, we can provide a more granular picture of the relationship between diabetic HDL and CTC (Table 4). The CTC positive rate observed was not significantly different between the high and normal HbA1c groups. We considered that this finding was also possibly due to the limited number of cases with hyperglycemia and that this should be addressed in future, larger studies with adequate power. Furthermore, in the regression analysis to exclude these confounders of blood glucose, the history of diabetes and hypoglycemic agent treatment were also found to be irrelated with CTCs. We hypothesized that a hyperglycemic state may result in increased survival of CTCs in the peripheral blood. Additional analyses, including the clinical assessment of homeostatic models and tumor cell culture in the relevant environment, will be performed to further characterize these associations.
Additionally, previous studies that used the CellSearch System for the detection of CTCs have indicated that CTCs were positively correlated with a larger tumor size, intensified lymph node involvement, unfavorable histological grade and vascular invasion (6,20,21). In this study, the pathological type was an additional factor associated with the presence of CTCs. Most of the special subtypes of invasive breast cancer, such as invasive medullary carcinoma and invasive mucinous carcinoma, have been conventionally regarded as less aggressive with a more favorable prognosis (22). Paradoxically, this study showed that the rate of positive CTCs in patients with IDC was lower than that in patients with lobular and other pathological types (9.0% vs. 11.1%, 28.6%, P=0.020). This disparity further demonstrates that the histological type alone may not be sufficient to serve as an important prognostic factor for long-term postoperative surveillance (23).
Our work has some limitations that should be taken into consideration when interpreting these results. Firstly, only two patients with detectable CTCs were diagnosed with metastasis by imaging examinations during our study period. As our study is still ongoing, a recurrence analysis of the follow-up results was not available. Secondly, for those patients with elevated blood glucose, it is probable that the failure to discover any correlations between CTCs, HDL and HbA1c is due to the limited sample size of our study. A more general limitation is that the CellSearch system is based on the detection of EpCAM and the more aggressive CTC classes are likely to undergo the epithelial-mesenchymal transition (EMT). During this process, CTCs lose EpCAM expression and weaken cell-to-cell adhesion. This phenomenon is accompanied by a corresponding acquisition of mesenchymal phenotype (24). Therefore, EpCAM-based CTC detection methods such as the CellSearch might miss CTCs that tend to initiate micro-metastasis (25,26). Furthermore, CTCs detected by the CellSearch System are non-viable and cannot be recovered for ex vivo cell culture or downstream analysis. Currently, a new method has been developed using the NP-HBCTC-Chip that is able to efficiently isolate tumor cells from blood. These cells can be recovered without any significant damage (27).
Conclusions
This study provided clinical evidence for the application of CTCs in Chinese patients with non-MBC and revealed the associations of CTCs with metabolic variables. Our study demonstrated that blood glucose and HDL are associated with CTCs. This finding supports the notion that the metabolic dysregulation in postoperative breast cancer patients may denote a more severe disease burden.
Acknowledgements
We thank all the patients who participated in this study. Additionally, we would like to thank the organizers of the OOTR conference and the reviewers who helped to improve the paper. This work was supported by the Special Fund of Development of Technology by Guangdong Province (No. 2016A030313768), the Special Fund of Guangzhou Science and Technology Bureau (No. 201707010418), the National Natural Science Foundation of China (No. 81602645), and the Science and Technology Project of Guangdong Province (No. 2012A03040001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 2007;446:765–70. [PubMed] DOI:10.1038/nature05760
- Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406–14. [PubMed] DOI:10.1016/S1470-2045(14)70069-5
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–91. [PubMed] DOI:0.1056/NEJMoa040766
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420–30. [PubMed] DOI:10.1200/JCO.2005.08.140
- Jiang ZF, Cristofanilli M, Shao ZM, et al. Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann Oncol 2013;24:2766–72. [PubMed] DOI:10.1093/annonc/mdt246
- Janni WJ, Rack B, Terstappen LW, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res 2016;22:2583–93. [PubMed] DOI:0.1158/1078-0432.CCR-15-1603
- Hepp P, Andergassen U, Jager B, et al. Association of CA27.29 and circulating tumor cells before and at different times after adjuvant chemotherapy in patients with early-stage breast cancer — The SUCCESS Trial. Anticancer Res 2016;36:4771–6. [PubMed] DOI:10.21873/anticanres.11034
- Sparano JA. GS6-03 Circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage II-III breast cancer. 2017 San Antonio Breast Cancer Symposium 2017; Abstract GS6-03.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [PubMed] DOI:10.1016/j.cell.2011.02.013
- Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J Clin Oncol 2016;34:4203–16. [PubMed] DOI:10.1200/JCO.2016.68.4480
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [PubMed] DOI:10.1245/s10434-010-0985-4
- Gnant M, Thomssen C, Harbeck N. St.. Gallen/Vienna 2015: A brief summary of the consensus discussion. Breast Care (Basel) 2015;10:124–30. [PubMed] DOI:10.1159/000430488
- Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol 2005;6:103–11. [PubMed] DOI:10.1016/S1470-2045(05)01736-5
- Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol 2012;30:164–71. [PubMed] DOI:10.1200/JCO.2011.36.2723
- Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 2011;29:25–31. [PubMed] DOI:10.1200/JCO.2010.29.7614
- Fayanju OM, Hall CS, Bauldry JB, et al. Body mass index mediates the prognostic significance of circulating tumor cells in inflammatory breast cancer. Am J Surg 2017;214:666–71. [PubMed] DOI:10.1016/j.amjsurg.2017.06.005
- Huang X, He D, Ming J, et al. High-density lipoprotein of patients with breast cancer complicated with type 2 diabetes mellitus promotes cancer cells adhesion to vascular endothelium via ICAM-1 and VCAM-1 upregulation. Breast Cancer Res Treat 2016;155:441–55. [PubMed] DOI:10.1007/s10549-016-3696-0
- Crujeiras AB, Diaz-Lagares A, Carreira MC, et al. Oxidative stress associated to dysfunctional adipose tissue: a potential link betweenobesity, type 2 diabetes mellitus and breast cancer. Free Radic Res 2013;47:243–56. [PubMed] DOI:10.3109/10715762.2013.772604
- Pan B, Yu B, Ren H, et al. High-density lipoprotein nitration and chlorination catalyzed by myeloperoxidase impair its effect of promoting endothelial repair. Free Radic Biol Med 2013;60:272–81. [PubMed] DOI:10.1016/j.freeradbiomed.2013.02.004
- Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106:pii: dju066. [PubMed] DOI:10.1093/jnci/dju066
- Sandri MT, Zorzino L, Cassatella MC, et al. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol 2010;17:1539–45. [PubMed] DOI:10.1245/s10434-010-0918-2
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer 2005;93:1046–52. [PubMed] DOI:10.1038/sj.bjc.6602787
- Kwast AB, Groothuis-Oudshoorn KC, Grandjean I, et al. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res Treat 2012;135:271–80. [PubMed] DOI:10.1007/s10549-012-2160-z
- May CD, Sphyris N, Evans KW, et al. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res 2011;13:202. [PubMed] DOI:10.1186/bcr2789
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012;14:R15. [PubMed] DOI:10.1186/bcr3099
- Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012;12:178. [PubMed] DOI:10.1186/1471-2407-12-178
- Park MH, Reátegui E, Li W, et al. Enhanced isolation and release of circulating tumor cells using nanoparticle binding and ligand exchange in a microfluidic chip. J Am Chem Soc 2017;139:2741–9. [PubMed] DOI:10.1021/jacs.6b12236
