Down-staging depth score to predict outcomes in locally advanced rectal cancer achieving ypI stage after neoadjuvant chemo-radiotherapy versus de novo stage pI cohort: A propensity score-matched analysis
Introduction
Preoperative concurrent chemo-radiotherapy (CRT) and total mesorectal excision (TME) have become the standard treatment of local advanced rectal cancer (LARC). Literatures reported that down-staging, especially yp0–I stage, after CRT was a significant prognostic indicator for long-term oncologic outcome (1-4). Our previous study showed that stage yp0–I patients after CRT achieved good overall survival (OS) with low relapse rate (5). However, doubt remains whether the local recurrence and distant metastasis rate can be comparable to that of early-stage rectal cancer treated primarily with TME. In the present study, we selected rectal cancer patients of ypT1–2N0 after CRT and maintenance chemotherapy and de novo pT1–2N0 from database of a prospective trial to explore the long-term results of the two groups of patients with propensity score-matched analysis.
Materials and methods
Patents selection
All patients with previously-untreated and histologically-proven rectal adenocarcinoma without distant metastasis treated at the Department of Radiation Oncology, National Cancer Center between January 1, 2008 and December 31, 2013 were selected from a prospectively maintained database. Patients underwent standard staging procedures with routine physical examination, and computed tomography (CT) scans of the chest, abdomen and pelvis. Magnetic resonance imaging (MRI) of the pelvis was required for primary tumor staging. Colorectal endoscopy was used to confirm the primary lesion to be below 12 cm from the anal verge. Patients in stage ypI group had clinical stage T3/4 and/or N+ (AJCC 2010) before the surgery, and pathology confirmed ypT1–2N0 disease afterwards. Stage pT1–2N0 patients, who received surgery alone, were included in the de novo stage pI group.
Treatment
Radiotherapy target volumes included primary tumor, mesorectal area, presacral space and pelvic sidewall that encompassed internal iliac lymph nodes and obturator lymph node region but not the external iliac lymphatic drainage area. Three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) [with 95% planning target volume (PTV) receiving 45.0–50.4 Gy in 1.8–2.0 Gy per fraction] was administered. Capecitabine (CAP) of 1,650 mg/(m2·d) alone, or CAP 1,300 mg/(m2·d) with oxaliplatin 50 mg/(m2·week) (CAPOX) regimen, was given concurrently with radiotherapy. Surgery was via total mesorectum excision (TME) with R0 resection, and was performed 4–8 weeks after the completion of CRT. Postoperative adjuvant chemotherapy was not mandatory. “Full-dose” adjuvant chemotherapy was defined as: FOLFOX regimen chemotherapy for 9 cycles, XELOX regimen for 6 cycles, or single-agent regimen being maintained for at least 6 months.
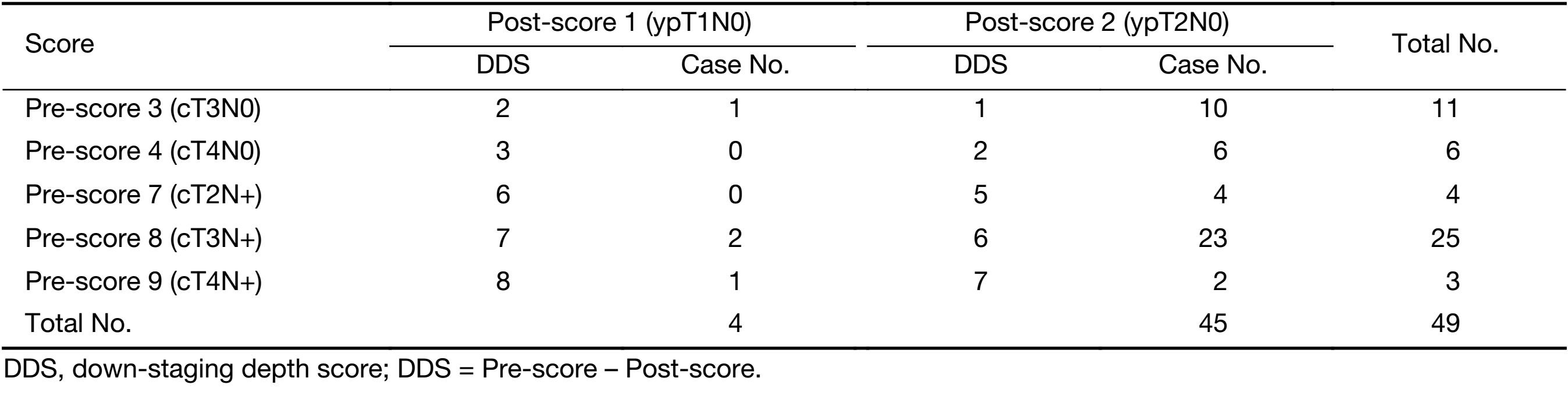
Down-staging depth score (DDS)
DDS, a response evaluation method by using TNM staging system, was applied to patients in ypI group (5). Stages T0–4N0 were scored as 0 to 4 points, while stages T0–4N+ were scored 5 to 9 points, respectively. The score before surgery was evaluated per clinical staging, and the postoperative score was based on pathological findings. Hence, DDS = Pre-score – Post-score. A DDS value of 5 or more was used to signify significant down-staging, and used to assess its impact on long-term prognosis quantitatively.
Follow-up and endpoints
Follow-up assessment was repeated every 3–6 months. Physical examinations, chest radiographs, abdominal CT or ultrasonography, pelvic MRI or CT scan, and hematological examinations were performed. Endoscopic examination of the rectum was repeated every year. Disease-free survival (DFS) was defined as the time from surgery in pI group or start day of CRT in ypI group to recurrence. OS was defined as the time from surgery in pI group or start day of CRT in ypI group to death as a result of all causes. Local recurrence or distant metastasis should be confirmed by CT, MRI or biopsy. Probabilities of local regional control (LC), distant metastasis-free survival (DMFS), DFS and OS were determined actuarially via Kaplan-Meier (KM) methodology.
Statistical analysis
Since patients were not randomly assigned to either treatment group due to the retrospective nature of the analysis, propensity score matching (PSM) was used to determine the independent impact of factors on long-term oncologic outcomes. Factors considered included: gender, age, anatomic distance to anus, T stage, intravascular cancer embolus, mucinous adenocarcinoma, neuroendocrine composition and nerve invasion. First, logistic regression using these variables was performed to obtain the propensity score for each patient (defined as the probability to be assigned to stage pI or ypI group according to the individual profile of these covariates). Then, patients in stage pI or ypI group were matched according to the calculated propensity scores using a k nearest neighbors (KNN) algorithm with a threshold of c≤0.01. After matching, KM analysis for LC, DMFS, DFS and OS were performed and compared between the two groups using the log-rank test.
To investigate the impact of DDS, the subset of patients with DDS score ≥5 was selected for PSM. The LC, DMFS, DFS and OS comparisons between the two sub-groups were then repeated.
Statistical analysis was done using the IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA). A two-sided P<0.05 was considered significant.
Results
Overall, 449 patients diagnosed with either stage pI or ypI rectal cancer between 2008 and 2013 were included for the present analysis. Of these patients, 49 (10.9%) had stage ypI rectal cancer (Table 1). Table 2 summarizes the patients’ baseline characteristics for both groups, indicating relevant differences between the two. Patients in stage pI group were significantly older, less male gender, and had a greater frequency of clinical T1 rectal cancer than patients with stage ypI patients. Twenty-two (44.9%) patients in ypI group underwent adjuvant chemotherapy. The chemotherapy regimen was FOLFOX, XELOX or single-agent capecitabine regimen. No stage pI patient received chemotherapy.
Full table

Full table
Entire cohort prior to PSM
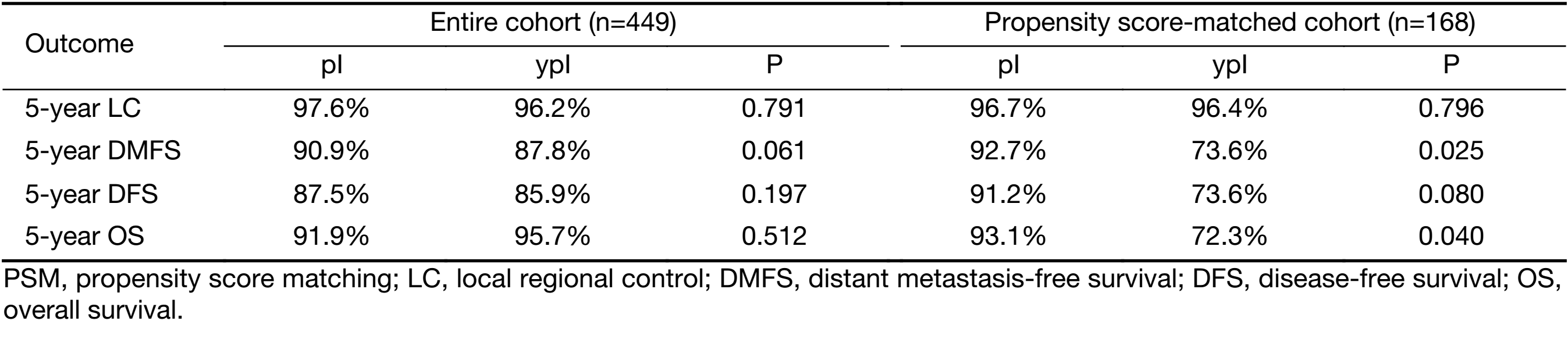
The median follow-up for survivors was 58 (range: 36–84) months in ypI group and 63 (range: 37–96) months in pI group, respectively. There were no significant differences in clinical outcomes between the two groups before PSM, although the characteristics baseline was different (Table 2). The 5-year local control rates were 97.6% [95% confidence interval (95% CI)]: 87.6–99.7) in stage pI group and 96.2% (95% CI: 87.2–96.4) in ypI group (P=0.791). The 5-year DMFS rates were 90.9% (95% CI: 74.8–93.3) and 87.8% (95% CI: 72.1–89.9) in the pI and ypI groups, respectively (P=0.061). There were no differences between the pI and ypI groups in terms of 5-year DFS (87.5% vs. 85.9%, P=0.197) or OS rates (91.9% vs. 95.7%, P=0.512).
Propensity score-matched cohort
PSM resulted in 42 matched pairs (ypI:pI=1:3), for a total of 168 patients. Patient and tumor characteristics were not significantly different between groups of the matched pairs (Table 2), indicating that the matching procedure worked well. Stage pI group patients resulted in superior 5-year DMFS (92.7% vs.73.6%, P=0.025; Figure 1A) and OS rate (93.1% vs. 72.3%, P=0.040; Figure 1B) as compared with ypI group. The pI group showed a trend towards better 5-year DFS (91.2% vs. 73.6%, P=0.080; Figure 1C) as compared with ypI group. No LC difference between these two groups was seen (96.7% vs. 96.4%, P=0.796; Figure 1D) (Table 3).

Full table
Recurrence and metastasis
In terms of patterns of recurrence, ypI group tends to have higher propensity for distant metastasis than pI group (11.9% vs. 7.1%) (Table 4) while rates of locoregional recurrence were comparable (2.4% vs. 3.2%). In ypI group, 1 case occurred in local regional area, and 5 cases of distant metastasis including 1 case of rectal anastomotic recurrence accompanied by peritoneum lymph node metastasis, 3 cases of lung metastasis, and 1 case of liver metastasis (Table 4). Five patients died during the follow-up period, 4 died of rectal cancer, and 1 died of non-cancer specific disease. Median DMFS time of pI and ypI group was 17.1 and 10.7 months, respectively. All patients with recurrence and metastasis received salvage treatment. After salvage therapy for relapse, the median survival time was similar in pI and ypI groups (30.7 vs. 29.5 months, P=0.808).

Full table
DDS favor cohort
In the ypI group, patients with DDS≥5 were selected and PSM was performed again according to sex, age and T stage. It resulted in 18 matched (ypI:pI=1:3) pairs, for a total of 72 patients. Patient and tumor characteristics were not significantly different between the two groups after PSM (Table 5). Of the DDS-favorable patients, there were 2, 13 and 3 with clinical stage IIIa, IIIb and IIIc, respectively. The 5-year clinical outcomes of ypI and pI groups in terms of LC (100% vs. 95.8%, P=0.384), DMFS (100% vs. 94.4%, P=0.368), DFS (100% vs. 92.2%, P=0.277) and OS (100% vs. 95.0%, P=0.458) were all similar.

Full table
Discussion
The present study compared the long-term prognosis of ypI rectal cancer and early rectal cancer treated with de novo surgery that resulted in pI status. The data were derived from a prospective phase II study in our single center that safeguarded the study integrity and ensured adequate follow-up rate. The results showed that the prognosis of stage ypI rectal cancer was good, with 5-year DFS and OS rates reaching 85.9% and 95.7%, respectively. However, actuarial survival analysis after PSM indicated that the distant metastasis rate of ypI group at 5 years was 26.4%, which was significant higher than 7.3% of pI stage rectal cancer (P=0.025). In DDS subgroup analysis, ypI group patients with DDS score ≥5 achieved very good outcomes of LC, DMFS, DFS and OS, which were comparable with early stage pI rectal cancer.
Approximately 40%–60% LARC patients could achieve down-staging after neoadjuvant CRT, which translates into long-term favorable oncologic outcomes (3,6,7). Our previous study showed that stage yp0–I patients attained 96% OS, with low relapse rate of 7%. Although it is widely acknowledged that the postoperative pathologic stages using TNM terminologies between neoadjuvant (i.e. “yp”) and de novo (i.e. “p”) settings are different in terms of their respective clinical meanings (8-10), few studies have specifically compared outcome differences in early stage rectal cancer between post-CRT patients and those undergoing TME surgery. Several studies demonstrated that, in comparison with the patients in stage pI, those in ypI group had several risk factors for poor oncologic outcomes such as higher carcinoembryonic antigen (CEA) level, more advanced T stage, and poorer histological differentiation (11,12). However, the patients in ypI group did not exhibit a higher disease progression rate or cancer-related death than those in pI group. Reerink et al. also showed that the prognoses for patients with initially unresectable rectal tumor down-staged to pT2 and those with primary resectable cancer with the same T classification are similar (9). In the study by Du et al., patients with early-stage rectal cancer were selected to undergo radical surgery as a control group, and the results demonstrated that post-CRT early stage rectal cancer has no significant different in prognosis (13). Unlike previously reported results, the present study indicated that, although the prognosis of patients with ypI stage LARC was good, the probability of distant metastasis was higher than that of de novo early-stage rectal cancer. This observation came from PSM, a method which could help reduce the selection bias between two study cohorts, making the results closer to that of a randomized controlled study. It thus suggests that, in some cases LARC are still prone to distant metastasis even with high LC after neoadjuvant CRT. Therefore, how to screen for patients with rectal cancer of different biological behaviors based on molecular phenotypes or clinical features has become an avidly-pursued research topic.
In recent years, studies of metastatic colorectal cancer using cytotoxic chemotherapy and targeted agents have revealed that the depth of down-staging impacted prognosis, and parameters such as early tumor shrinkage (ETS) and depth of response (DpR) could effectively evaluate response and predict long-term survival (14-16). ETS was defined as tumor shrinkage of 10% to 30% or more at the first evaluation after treatment. DpR was defined as the percentage of tumor shrinkage when the lesion’s maximum diameter or volume reached the lowest value as compared to the baseline tumor size. Heinemann et al. assumed that DpR, which may be associated with specific anti-proliferative agents, as a clinical response evaluation index could predict PFS and OS and provide a basis for subsequent treatment strategy (17). de Campos-Lobato et al. found that down-staging did not significantly improve the LC, DFS and OS for stage cII rectal cancer, whereas it yielded good outcome in patients with stage cIII LARC. It thus suggested that the predictive value of down-staging for patients with more advanced pretreatment stage may be better (18). Neoadjuvant rectal (NAR) scoring system, reported in George’s literature, was based on three indicators: pre-treatment clinical T stage, postoperative pathologic T and N stage, and one by using the nomogram formula published by Valentini (19,20). Several studies reported that the down-staging depth was associated with prognosis (19,21). It was used to analyze the results in NSABP R-04 study, with the conclusion that NAR could be used to predict OS (P<0.001) with relative ease. Therefore, the author illustrated that NAR could be a new effective prognostic factor, as well as pCR and tumor regression grade (TRG) classification. Our previous study concluded that DDS, an indicator of staging dynamic changes, was associated with DMFS, DFS and OS, and that the ability to predict prognosis was superior to pCR and TRG grades (5). In our study, DDS method is similar to the NAR scoring system, but is simpler to obtain, and the preoperative N-staging was considered. With the considerations of ETS, DpR, NAR and the results of this study, the changes of tumor extent at specific time points could provide more information about the lesion’s biological behavior and treatment sensitivity, thereby setting a new trend in evaluating response. Recent imaging studies have also focused on the evaluation of prognostic values based on dynamic changes of tumor dimensions (22,23). Therefore, predictive indicators based on response should take into account the overall depth of remission before and after treatment. Simply relying on qualitative evaluation of pre- or post-treatment tumor status may not be comprehensive or accurate enough to provide prognostication. The results of our study also found that DDS was an effective prognostic indicator, enabling accurate selection of patients with good long-term outcomes. The LC, DMFS, DFS and OS of DDS-favorable patients were comparable to those of early rectal cancer cohort.
The conclusion in this paper was derived from observation based on prospectively maintained database, and PSM method was used to compare two cohorts to reduce potential bias. The goal of the present study is to determine the prognostic parameters for LARC after neoadjuvant CRT in order to guide subsequent treatment strategy. The MERCURY study had shown good correlation between post-treatment MRI disease status and tumor histopathology, resulting in specific diagnostic guideline (24). MRI has an accuracy of 88% in staging rectal disease after neoadjuvant treatment, therefore could accurately determine the pathological regression extent and dictate the following surgical treatment, even accurately selecting cases suitable for watch-and-wait strategy based on results of the present study.
This study does have its limitations. Firstly, the number of patients was limited, especially in the neoadjuvant treatment group. As all cases were selected from a phase II database and the proportion of down-staging was not high, the number of stage ypI cohort was relatively small. Therefore, despite the use of PSM method, the restrictive number of cases might still affect the long-term survival and DDS analysis results. Secondly, we did not include the analysis of postoperative chemotherapy for LARC patients due to its inconsistent effects. Previous studies have reported that the value of chemotherapy after neoadjuvant CRT remains uncertain. PSM analysis from study of Park et al. concerning 1,016 ypT0–2N0 stage patients from 10 centers showed that adjuvant chemotherapy was not associated with improvement in 5-year RFS (P=0.62) (25). In addition, since all patients in pI group did not receive adjuvant chemotherapy, we did not take into account the influence of adjuvant chemotherapy when analyzing with PSM methodology.
Conclusions
Local control was comparable in both de novo pI stage cohort and LARC group achieving ypI stage. However, distant metastasis was more frequent in down-staged LARC patients as compared to early-stage cases. The overall prognosis of patients with sufficient down-staging of tumor extent was relatively good. Furthermore, the clinical stage after concurrent chemoradiation can achieve a high rate of correlation with postoperative pathology. Therefore, this study provides support for appropriate use of prognostic factors in patients with LARC in order to guide further treatment strategy after neoadjuvant chemoradiation.
Acknowledgements
This work is supported by Natural Science Foundation of China (No. 81773241); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2017-I2M-1-006).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [PubMed] DOI:10.1056/NEJMoa040694
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. [PubMed] DOI:10.1056/NEJMoa060829
- Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 2007;109:1750–5. [PubMed] DOI:10.1002/cncr.22625
- Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–46. [PubMed] DOI:10.1016/S1470-2045(17)30086-4
- Li N, Jin J, Yu J, et al. Prognostic factors in patients with stage yp0-I rectal cancer after preoperative concurrent chemoradiotherapy. Zhonghua Fang She Zhong Liu Xue Za Zhi (in Chinese) 2017;26:296–301. DOI:10.3760/cma.j.issn.1004-4221.2017.03.010
- Theodoropoulos G, Wise WE, Padmanabhan A, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum 2002;45:895–903. [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379–86. [PubMed] DOI:10.1200/JCO.2007.11.9685
- Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 2004;28:215–23. [PubMed]
- Reerink O, Verschueren RC, Szabo BG, et al. A favourable pathological stage after neoadjuvant radiochemotherapy in patients with initially irresectable rectal cancer correlates with a favourable prognosis. Eur J Cancer 2003;39:192–5. [PubMed]
- Jass JR, O’Brien MJ, Riddell RH, et al. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Virchows Arch 2007;450:1–13. [PubMed] DOI:10.1007/s00428-006-0302-6
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–94. [PubMed] DOI:10.1043/0003-9985(2000)124<0979:PFICC>2.0.CO;2
- Nissan A, Stojadinovic A, Shia J, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated by surgery alone. J Clin Oncol 2006;24:4078–84. [PubMed] DOI:10.1200/JCO.2006.06.2968
- Du CZ, Chen YC, Cai Y, et al. Oncologic outcomes of primary and post-irradiated early stage rectal cancer: a retrospective cohort study. World J Gastroenterol 2011;17:3229–34. [PubMed] DOI:10.3748/wjg.v17.i27.3229
- Mansmann UR, Laubender RP. Methodologic diligence is needed to define and validate tumor-size response metrics to predict overall survival in first-line metastatic colorectal cancer. J Clin Oncol 2013;31:4373–4. [PubMed] DOI:10.1200/JCO.2013.51.2954
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065–75. [PubMed] DOI:10.1016/S1470-2045(14)70330-4
- Piessevaux H, Buyse M, De Roock W, et al. Radiological tumor size decrease at week 6 is a potent predictor of outcome in chemorefractory metastatic colorectal cancer treated with cetuximab (BOND trial). Ann Oncol 2009;20:1375–82. [PubMed] DOI:10.1093/annonc/mdp011
- Heinemann V, Stintzing S, Modest DP, et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 2015;51:1927–36. [PubMed] DOI:10.1016/j.ejca.2015.06.116
- de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Downstaging without complete pathologic response after neoadjuvant treatment improves cancer outcomes for cIII but not cII rectal cancers. Ann Surg Oncol 2010;17:1758–66. [PubMed] DOI:10.1245/s10434-010-0924-4
- George TJ, Jr. Neoadjuvant Rectal (NAR) Score: a new surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Rep 2015;11:275–80. [PubMed] DOI:10.1007/s11888-015-0285-2
- van Gijn W, van Stiphout RG, van de Velde CJ, et al. Nomograms to predict survival and the risk for developing local or distant recurrence in patients with rectal cancer treated with optional short-term radiotherapy. Ann Oncol 2015;26:928–35. [PubMed] DOI:10.1093/annonc/mdv023
- Rosello S, Frasson M, Garcia-Granero E, et al. Integrating downstaging in the risk assessment of patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: validation of valentini's nomograms and the neoadjuvant rectal score. Clin Colorectal Cancer 2018;17:104–12.e2. [PubMed] DOI:10.1016/j.clcc.2017.10.014
- Neri E, Guidi E, Pancrazi F, et al. MRI tumor volume reduction rate vs tumor regression grade in the pre-operative re-staging of locally advanced rectal cancer after chemo-radiotherapy. Eur J Radiol 2015;84:2438–43. [PubMed] DOI:10.1016/j.ejrad.2015.08.008
- Li N, Dou L, Zhang Y, et al. Use of sequential endorectal US to predict the tumor response of preoperative chemoradiotherapy in rectal cancer. Gastrointest Endosc 2017;85:669–74. [PubMed] DOI:10.1016/j.gie.2016.06.042
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753–60. [PubMed] DOI:10.1200/JCO.2011.34.9068
- Park IJ, Kim DY, Kim HC, et al. Role of adjuvant chemotherapy in ypT0-2N0 patients treated with preoperative chemoradiation therapy and radical resection for rectal cancer. Int J Radiat Oncol Biol Phys 2015;92:540–7. [PubMed] DOI:10.1016/j.ijrobp.2015.02.020
