A nomogram to predict prognosis for gastric cancer with peritoneal dissemination
Introduction
Despite the decrease in the incidence of gastric cancer over the past few decades, it remains the second most common cause of cancer-related death among carcinomas in the world, with 738,000 deaths globally, mainly in Latin America, Eastern Europe and Eastern Asia (1-4). In many countries, including China, gastric cancer patients are diagnosed at a relatively advanced stage (5). In addition, 30%−60% of gastric cancer patients were found to have peritoneal dissemination at the time of diagnosis and have a median survival time of only 3−6 months (6).
Despite the progress in surgery, chemotherapy and radiotherapy, the prognosis of gastric cancer is still unsatisfactory (7). Recently, the molecular targeting of genes, such as human epidermal growth factor receptor 2 (Her2), has been the focus of many gastric cancer investigations, and several molecular targeting agents have been approved for use in clinic practice due to their beneficial effects on the short-term survival of gastric cancer patients with or without peritoneal dissemination (8,9). However, the effects of Trastuzumab on the subgroup of patients with peritoneal dissemination have been unclear.
In the clinical setting, the 7th version of the TNM Staging System of the American Joint Committee on Cancer (AJCC) is used to stage gastric cancer patients and predict their prognosis. However, other important clinicopathological features, including the gross type and patient age, among others, are not taken into consideration in this system. We and other investigators have found that gastric cancer patients may have a different prognosis even when they are classified in the same stage. Above all, a more reliable tool is needed to predict the prognosis of patients so better treatments can be administered. A nomogram is an efficient tool to quantify risks by combining all known prognostic factors using statistical software and has demonstrated practical use in the diagnosis of several diseases. There are already a few nomograms that have been developed to estimate the prognosis of gastric cancer, for example, the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram for gastric cancer, which is used to predict the 5-year and 9-year disease-specific survival (DSS) after an R0 resection without any other therapy, has been widely validated and proven to be accurate (10-21). We have observed that some patients with gastric cancer with peritoneal dissemination (GCPD) achieved a better prognosis after receiving palliative gastrectomy, chemotherapy or hyperthermic intraperitoneal chemotherapy (HIPEC). However, it is difficult to identify these patients clinically. In this study, we tried to develop a nomogram model to predict the prognosis of gastric cancer patients with peritoneal dissemination by months of survival, for the purpose of screening out appropriate patients for more aggressive treatment.
Materials and methods
Ethics statement
All the patients provided written informed consent for their information to be stored in the hospital database of the Sixth Affiliated Hospital of Sun Yat-sen University and the Sun Yat-sen University Cancer Center. We obtained separate consent for the use of this information for research. Study approval was obtained from the independent Ethics Committees of the Sixth Affiliated Hospital of Sun Yat-sen University and the Sun Yat-sen University Cancer Center. This study was performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Patient inclusion and exclusion criteria
The inclusion criteria were as follows: 1) histologically proven adenocarcinoma of the stomach with synchronous peritoneal dissemination at the time of surgery or biopsy; and 2) no other synchronous or metachronous cancers. The exclusion criteria were as follows: 1) incomplete or important data censored; 2) patients with mental disorders or severe dysfunction of the liver and/or kidneys; or 3) no pathological diagnosis of peritoneal dissemination.
Peritoneal seeding status
We classified peritoneal seeding status according to the first English edition of the Japanese Classification of Gastric Carcinoma (22). The classification was as follows: P0, no peritoneal seeding; P1, disseminated metastasis to the region directly adjacent to the peritoneum of the stomach (above the transverse colon, including the greater omentum); P2, several scattered metastases to the distant peritoneum and ovarian metastasis only; and P3, numerous metastases to the distant peritoneum.
Follow-up
After treatment, the patients were monitored every month for the first year, every 3 months for the second year, and every 6 months thereafter, with regular follow-up assessments. We always followed up using telephone interviews, a follow-up letter, a short message platform and an email.
End points
In our research, the end point is the overall survival of patients with peritoneal dissemination. We followed up all the patients until June 2017 or the death of patients.
Statistical analysis
All data were analyzed using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). The clinicopathological parameters between the development cohort and the validation cohort were analyzed using a Chi-square test or Fisher’s exact test. Then, we performed a Kaplan-Meier analysis and Cox regression analysis to evaluate the independent prognostic factors for overall survival of the gastric cancer patients with peritoneal dissemination.
A nomogram was developed as a tool to predict the prognosis of gastric cancer patients with peritoneal dissemination by the months of survival. It graphically presents the patient prognosis. The nomogram and the calibration curve were displayed using the package of Regression Modeling Strategies in R. The predictive performance of this model was evaluated in the test group using the concordance index (C-index) (11). P<0.05 was considered statistically significant.
Results
Patient characteristics
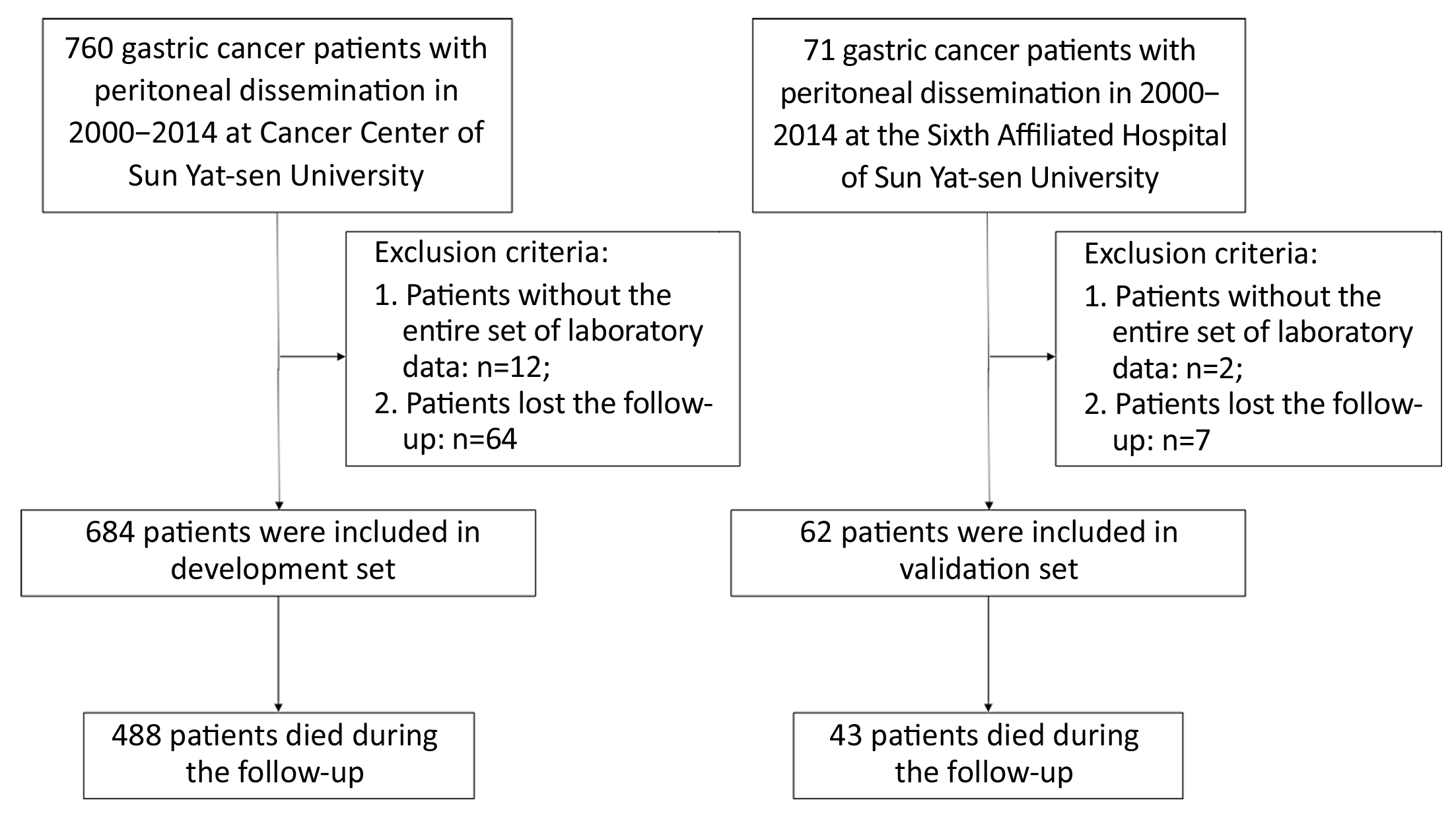
From January 2000 to December 2014, a total of 746 patients with histologically diagnosed GCPD were included in the present study: 684 consecutive patients from Sun Yat-sen University Cancer Center comprised the development cohort, and the other 62 consecutive patients from the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China comprised the validation cohort. The flow chart is shown in Figure 1. All cases of peritoneal dissemination were proven clinicopathologically at surgery. The clinicopathological factors for the development set and validation set are shown in Table 1. Among the 684 patients from Sun Yat-sen University Cancer Center, 31 patients received gastrectomy +perioperative chemotherapy; 195 patient received gastrectomy + postoperative chemotherapy; 70 patients received gastrectomy only; 31 patients received bypass surgery + postoperative chemotherapy; 37 patients received bypass surgery only; another 226 patients received palliative chemotherapy only, and the remaining 94 patients did not receive any therapy. In addition, among the 62 patients from the Sixth Affiliated Hospital of Sun Yat-sen University, 3 patients received gastrectomy +perioperative chemotherapy; 45 patient received gastrectomy + postoperative chemotherapy; 3 patients received bypass surgery only; another 3 patients received palliative chemotherapy only; and the remaining 8 patients did not receive any therapy. Regarding hyperthermic intraperitoneal perfusion, 24 patients in the development group and 16 patients in the validation group accepted this treatment after surgery. None of the patients received targeted chemotherapy according to our records. Patient survival was measured from the diagnosis of peritoneal dissemination. The median follow-up time and median survival for all patients were 12.5 (range: 1−152) months and 11.5 [95% confidence interval (95% CI): 10.4−12.6] months, respectively.

Full table
Univariate and multivariate analyses (Cox’s regression) of gastric cancer patients in development set
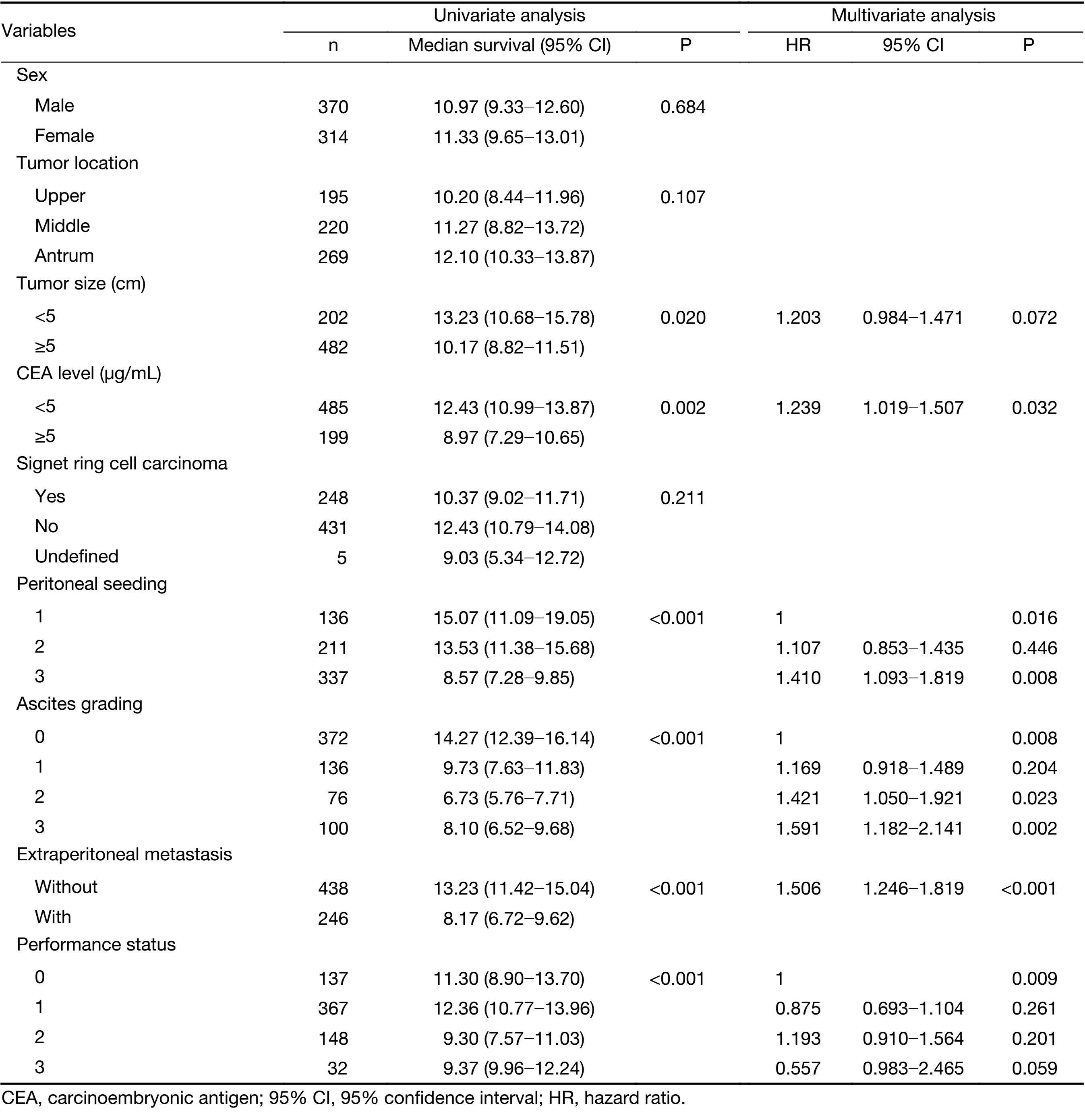
Univariate analysis showed that tumor size, serum carcinoembryonic antigen (CEA) level, ascites grading, presence of extraperitoneal metastasis, seeding status and performance status were prognostic factors for these patients. We included these clinicopathological factors identified as prognostic factors in the univariate analysis (P<0.05) for the Cox regression model. Multivariate analysis showed that serum CEA level, ascites grading, presence of extraperitoneal metastasis, seeding status and performance status were independent prognostic factors for gastric cancer patients with peritoneal dissemination in the development set. The results of the univariate and multivariate analyses are shown in Table 2.
Full table
Development and internal validation of nomogram model to predict prognosis of gastric cancer patients with peritoneal dissemination
Along with the visualized tool provided by the nomogram, we then used the Cox regression model to predict the prognosis by months of survival of the gastric cancer patients with peritoneal dissemination. The nomogram model is shown in Figure 2.
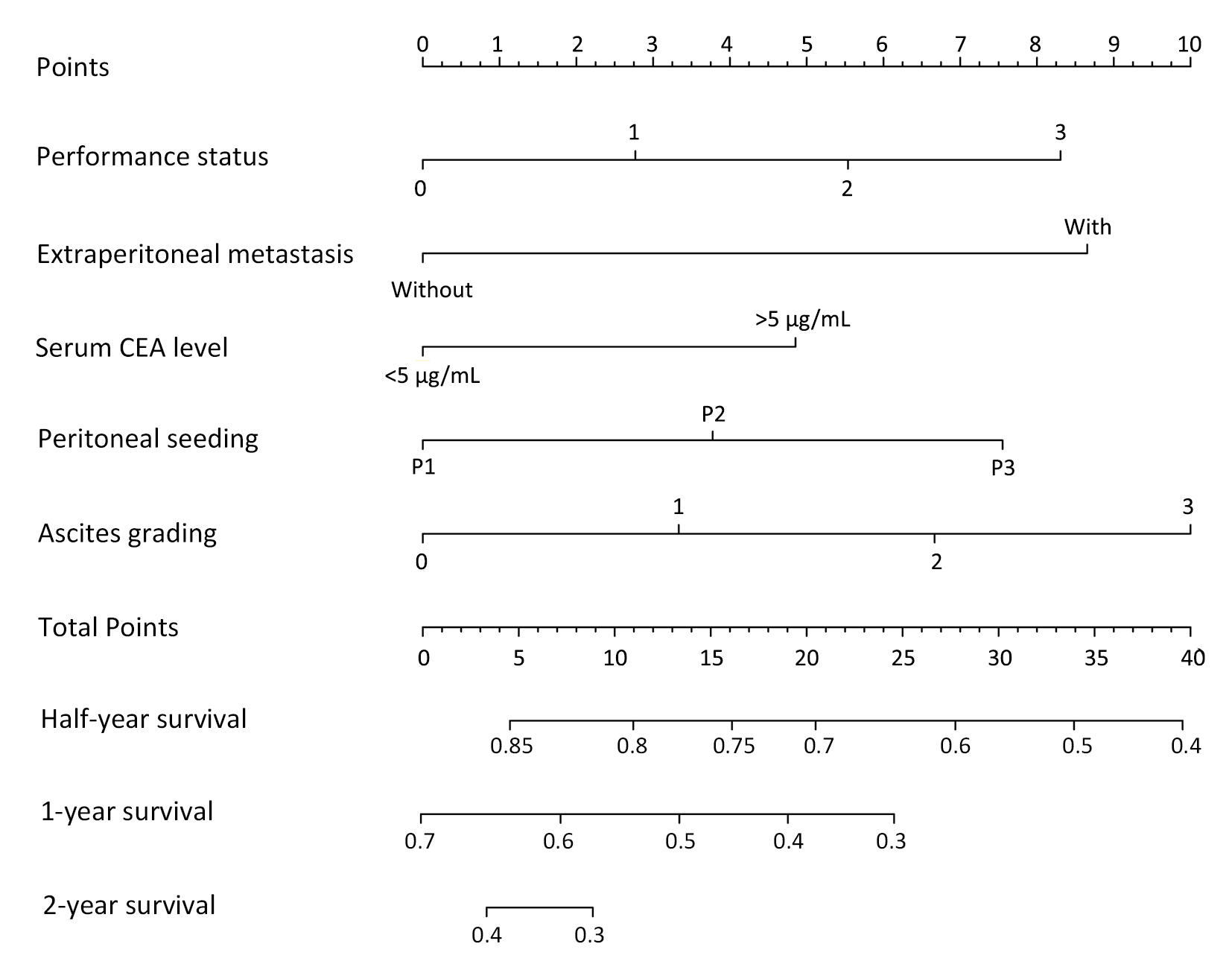
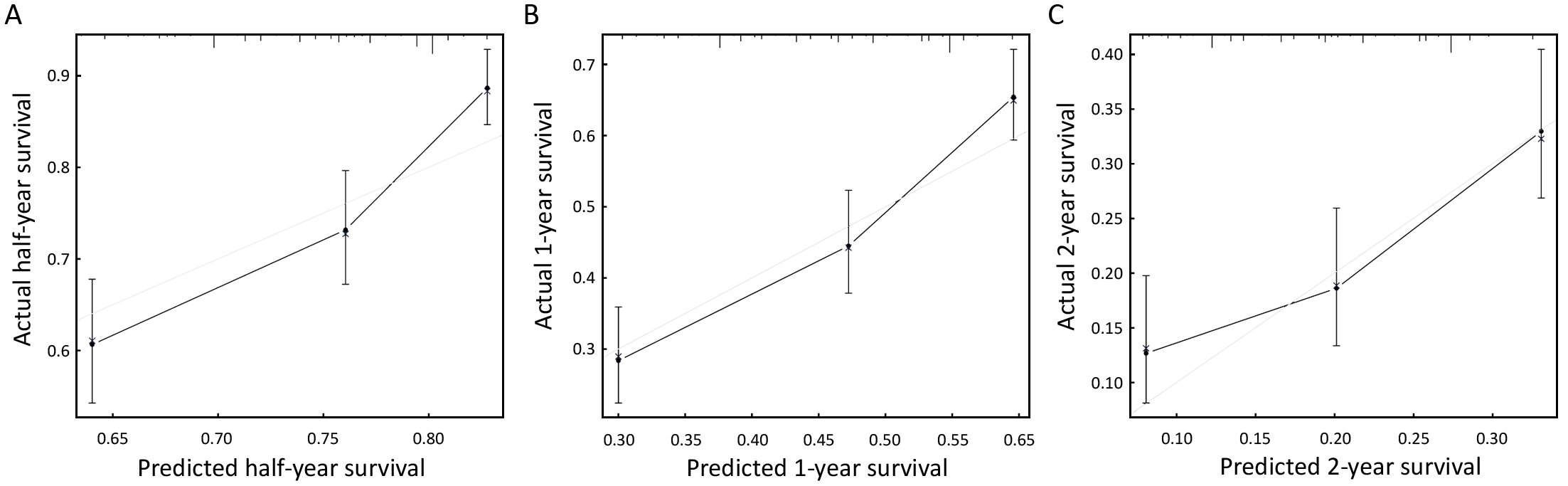
We performed an internal validation to validate this nomogram and found that the concordance index was 0.641, which closely corresponded to the actual survival. The internal calibration curves for half-, 1- and 2-year survival are shown in Figure 3. As shown in
Figure 3, the black line represents the predicted values of the nomogram, while the gray line represents the actual values. The less discrepant they are, the more precise the predictive capability of the model is. For the internal calibration, the black lines fluctuated above and below the gray lines, to identify a reliable predictive capability of the nomogram.
External validation of nomogram model of gastric cancer patients from the Sixth Affiliated Hospital of Sun Yat-sen University
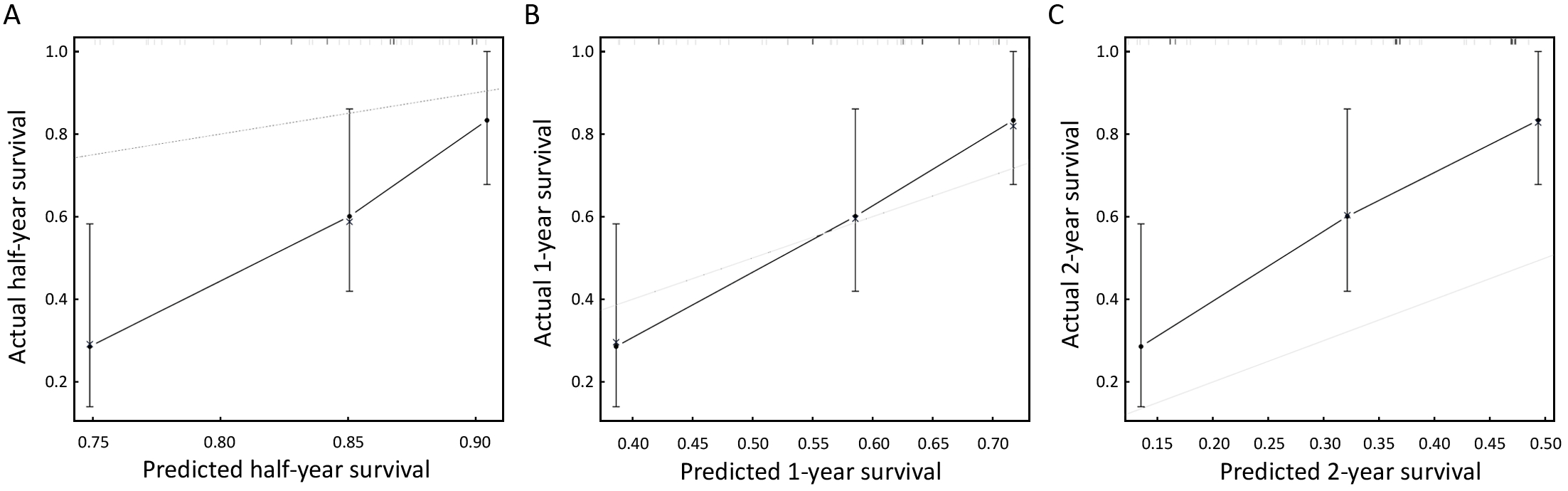
We used an external validation set, consisting of 62 gastric cancer patients from the Sixth Affiliated Hospital of Sun Yat-sen University, to characterize the discrimination of the newly developed nomogram model. The C-index was 0.709, and the external calibration curves for half-, 1- and 2-year survival are shown in Figure 4. As shown in Figure 4, the goodness of fit indicated relatively satisfactory predictive values in the external validation, even though the predicted half-year survival and the predicted 2-year survival were not as fit as the predicted 1-year survival.
Discussion
Peritoneal dissemination is one of the most common forms of metastasis for gastric cancer. Palliative chemotherapy has been the main treatment administered to gastric cancer patients with peritoneal dissemination. In the past few decades, researchers have devoted efforts toward the development of new treatments for gastric cancer patients with peritoneal dissemination, including radiotherapy, chemotherapy, and molecular targeted drugs (23). Unfortunately, the results of clinical trials have been unsatisfactory, although some patients were reported to experience prolonged survival after they received intraperitoneal perfusion of chemotherapy or combination treatment with chemoradiotherapy (24). Clinically, it is important to predict the prognosis of gastric cancer patients with peritoneal dissemination to ensure that they receive the most optimal treatment.
Currently, the 7th Edition of the AJCC Staging System is used clinically to predict the survival of gastric cancer patients. However, this system places all the gastric cancer patients with peritoneal dissemination into stage IV. In a previous report, it was found that the patient age, tumor location, total number of lymph nodes retrieved, postoperative recurrence, adjuvant radio/chemotherapy, the Lauren classification, etc. were related to the prognosis of gastric cancer patients (10-14,16-18,25-27). In our present study, we identified serum CEA level, ascites grading, presence of extraperitoneal metastasis, seeding status, and performance status as independent prognostic risk factors for GCPD patients by a Cox regression model. In our research, all the patients were gastric cancer patients with synchronous peritoneal dissemination. The prognostic influence of some proven clinicopathological factors, Lauren type, lymph node metastasis, lymphovascular and perineural invasion might be inconsequential in the presence of distant metastasis. In our previous study, we found that palliative gastrectomy can prolong the survival of GCPD patients without extraperitoneal metastasis when combined with more than five cycles, and particularly more than eight cycles, of first-line chemotherapy (28-37). In another study, it was shown that preoperative or postoperative chemotherapy on the basis of S-1, alone or combined with other chemotherapeutic agents such as cisplatin (SP therapy), paclitaxel or oxaliplatin as first-line chemotherapy can prolong patient survival, with one patient still alive three years after
chemotherapy (38-40).
In support of the practicality of our model, the C-indexs were 0.641 and 0.709 in the internal and external validation subsets. This finding means that the model was still not precise enough and needed to be optimized, which might be related to the following reasons. First, the treatment strategies in this group of patients were complex. As aforementioned, there were 6 treatment strategies in our data: 1) gastrectomy + perioperative chemotherapy; 2) gastrectomy + postoperative chemotherapy; 3) gastrectomy only; 4) bypass surgery + postoperative chemotherapy; 5) bypass surgery + postoperative chemotherapy; 6) palliative chemotherapy only; and 7) no therapy. In addition, some patients received HIPEC. Different treatments may affect the prognoses of the patients. Second, we only included the preoperative variables to construct the model to make it fit clinically. However, the effects of the treatments were disregarded in the model. Finally, this study had bias because it was a retrospective study; moreover, for the validation group, more patients from multiple centers will be needed for external validation to fully examine this model. However, the calibration was unsatisfactory, which we believe may be related to the different treatment strategies accepted in the validation group compared with those in the development group. The percentage of patients in the validation group who received chemotherapy was lower than that of the development group. This observation may account for the lower fitness of the predicted half-year survival and of the predicted 2-year survival. In addition, the number of patients in the validation group is too small, which also may have caused bias in the calibration curve. In conclusion, we have reasons to believe that the nomogram we constructed has prognostic potential accordingly.
We therefore believe that this model can be used to predict the prognosis of GCPD. Chemotherapy is currently the mainstay of treatment for GCPD patients. However, some of these patients were shown to have a better prognosis under comprehensive therapy that included surgery and other approaches. This model could be practical for suggesting the optimal clinical treatment for these patients. For example, for the patients predicted to have a better prognosis, aggressive treatments including surgery or HIPEC should be considered. For the patients predicted to have a poor prognosis, only palliative therapy should be administered. We are the first to identify possible risk factors for patients with peritoneal dissemination in a nomogram and believe that this model should permit individualized survival prediction and provide better treatment allocation than the existing systems. We believe that our nomogram may assist surgeons in selecting the appropriate treatment for gastric cancer patients with regard to the probability of a survival benefit.
As shown in Table 1, there were several clinicopathological factors that differed significantly between the development group and the validation group, such as signet-ring cell carcinoma, peritoneal seeding, extraperitoneal metastasis, performance status, and palliative chemotherapy. Among them, peritoneal seeding, extraperitoneal metastasis and performance status were included in the nomogram model, which may demonstrate the validity of the model for two different groups of patients. However, the ratio of the palliative chemotherapy group may cause a deviation of the prediction from the model because different treatment strategies may cause survival differences. Another group of patients who had treatment strategies similarly proportional to those of the development group may be needed to validate the accuracy of the model.
There are some limitations associated with our study. First, although we constructed a robust nomogram model for prognostic prediction, more patients from multiple centers will be needed for external validation to fully examine this model. In addition, we removed some patients who had been lost to follow-up or discontinued follow-up for various reasons. Other patients were also removed because of missing data, such as tumor size, precise pathological staging and performance status. Second, the findings may not be generalizable to gastric cancer in Western countries.
Conclusions
We have constructed a nomogram to estimate the survival of gastric cancer patients with peritoneal dissemination. The internal and external validations showed that this model can effectively predict the prognosis of gastric cancer patients with peritoneal dissemination. However, the number of subjects included in the external validation set was too small. More data are needed to validate and modify this model.
Acknowledgements
This work was supported in part by a major special project grant from Guangzhou Health and Medical Collaborative Innovation (No. 15570006) and the Tianhe District Science and Technology Project, Guangdong, China (No. 201434KW020).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 2012;4:156–69. [PubMed] DOI:10.4251/wjgo.v4.i7.156
- Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel) 2013;5:48–63. [PubMed] DOI:10.3390/cancers5010048
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71. [PubMed] DOI:10.3322/caac.21235
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1–10. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.01.01
- Li Z, Li Z, Jia S, et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin J Cancer Res 2017;29:109–17. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.02.03
- Fushida S, Oyama K, Kinoshita J, et al. VEGF is a target molecule for peritoneal metastasis and malignant ascites in gastric cancer: prognostic significance of VEGF in ascites and efficacy of anti-VEGF monoclonal antibody. Onco Targets Ther 2013;6:1445–51. [PubMed] DOI:10.2147/OTT.S51916
- Yamaguchi H, Kitayama J, Ishigami H, et al. A patient with gastric cancer with peritoneal carcinomatosis treated with intraperitoneal chemotherapy who survived more than 5 years receiving repeated laparoscopic examinations: a case report. J Med Case Rep 2016;10:14. [PubMed] DOI:10.1186/s13256-016-0799-5
- Liu H, Zhang H, Shen Z, et al. Increased expression of CSF-1 associates with poor prognosis of patients with gastric cancer undergoing gastrectomy. Medicine (Baltimore) 2016;95:e2675. [PubMed] DOI:10.1097/MD.0000000000002675
- Izuishi K, Haba R, Kushida Y, et al. S-1 and the treatment of gastric cancer with peritoneal dissemination. Exp Ther Med 2011;2:985–90. [PubMed] DOI:10.3892/etm.2011.290
- Dikken JL, Baser RE, Gonen M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann Surg Oncol 2013;20:1623–30. [PubMed] DOI:10.1245/s10434-012-2723-6
- Zheng Z, Zhang Y, Zhang L, et al. A nomogram for predicting the likelihood of lymph node metastasis in early gastric patients. BMC Cancer 2016;16:92. [PubMed] DOI:10.1186/s12885-016-2132-5
-
Song KY, Park YG, Jeon HM, et al. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer 2014;17:287–93.
[PubMed]
DOI:10.1007/s10120-013-0270-x>
- Kim Y, Spolverato G, Ejaz A, et al. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma. Ann Surg Oncol 2015;22:1828–35. [PubMed] DOI:10.1245/s10434-014-4230-4
- Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol 2014;25:1179–84. [PubMed] DOI:10.1093/annonc/mdu125
- Reim D, Novotny A, Eom BW, et al. External validation of an eastern Asian nomogram for survival prediction after gastric cancer surgery in a European Patient Cohort. Medicine (Baltimore) 2015;94:e2406. [PubMed] DOI:10.1097/MD.0000000000002406
- Lagarde SM, Reitsma JB, de Castro SM, et al. Prognostic nomogram for patients undergoing oesophagectomy for adenocarcinoma of the oesophagus or gastro-oesophageal junction. Br J Surg 2007;94:1361–8. [PubMed] DOI:10.1002/bjs.5832
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834–40. [PubMed] DOI:10.1200/JCO.2012.41.8343
- Eom BW, Ryu KW, Nam BH, et al. Survival nomogram for curatively resected Korean gastric cancer patients: multicenter retrospective analysis with external validation. PLoS One 2015;10:e0119671. [PubMed] DOI:10.1371/journal.pone.0119671
- Chen D, Jiang B, Xing J, et al. Validation of the Memorial Sloan-Kettering Cancer Center nomogram to predict disease-specific survival after R0 resection in a Chinese gastric cancer population. PLoS One 2013;8:e76041. [PubMed] DOI:10.1371/journal.pone.0076041
- Peeters KC, Kattan MW, Hartgrink HH, et al. Validation of a nomogram for predicting disease-specific survival after an R0 resection for gastric carcinoma. Cancer 2005;103:702–7. [PubMed] DOI:10.1002/cncr.20783
- Ashfaq A, Kidwell JT, McGhan LJ, et al. Validation of a gastric cancer nomogram using a cancer registry. J Surg Oncol 2015;112:377–80. [PubMed] DOI:10.1002/jso.23999
- Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma, First English edition. Tokyo: Kanehara & Co., Ltd, 1995.
- Ellison LM, Man Y, Stojadinovic A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis. Chin J Cancer Res 2017;29:86–92. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.01.10
- Yonemura Y, Kawamura T, Bando E, et al. Treatment results of peritoneal dissemination from gastric cancer by neoadjuvant intraperitoneal-systemic chemotherapy. Gan To Kagaku Ryoho (in Japanese) 2004;31:1723–6. [PubMed]
- Lagarde SM, ten Kate FJ, Reitsma JB, et al. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol 2006;24:4347–55. [PubMed] DOI:10.1200/JCO.2005.04.9445
- Barchi LC, Yagi OK, Jacob CE, et al. Predicting recurrence after curative resection for gastric cancer: External validation of the Italian Research Group for Gastric Cancer (GIRCG) prognostic scoring system. Eur J Surg Oncol 2016;42:123–31. [PubMed] DOI:10.1016/j.ejso.2015.08.164
- Liu K, Yang K, Wu B, et al. Tumor-infiltrating immune cells are associated with prognosis of gastric cancer. Medicine (Baltimore) 2015;94:e1631. [PubMed] DOI:10.1097/MD.0000000000001631
- Yang K, Liu K, Zhang WH, et al. The value of palliative gastrectomy for gastric cancer patients with intraoperatively proven peritoneal seeding. Medicine (Baltimore) 2015;94:e1051. [PubMed] DOI:10.1097/MD.0000000000001051
- Nie RC, Chen S, Yuan SQ, et al. Significant role of palliative gastrectomy in selective gastric cancer patients with peritoneal dissemination: a propensity score matching analysis. Ann Surg Oncol 2016;23:3956–63. [PubMed] DOI:10.1245/s10434-016-5223-2
- Tokunaga M, Terashima M, Tanizawa Y, et al. Survival benefit of palliative gastrectomy in gastric cancer patients with peritoneal metastasis. World J Surg 2012;36:2637–43. [PubMed] DOI:10.1007/s00268-012-1721-y
- Kunisaki C, Makino H, Takagawa R, et al. Impact of palliative gastrectomy in patients with incurable advanced gastric cancer. Anticancer Res 2008;28:1309–15. [PubMed]
- Hioki M, Gotohda N, Konishi M, et al. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg 2010;34:555–62. [PubMed] DOI:10.1007/s00268-010-0396-5
- Chen S, Li YF, Feng XY, et al. Significance of palliative gastrectomy for late-stage gastric cancer patients. J Surg Oncol 2012;106:862–71. [PubMed] DOI:10.1002/jso.23158
- Kwon IG, Cho I, Choi YY, et al. Risk factors for complications during surgical treatment of remnant gastric cancer. Gastric Cancer 2015;18:390–6. [PubMed] DOI:10.1007/s10120-014-0369-8
- Iwasa S, Nakajima TE, Nakamura K, et al. Systemic chemotherapy for peritoneal disseminated gastric cancer with inadequate oral intake: a retrospective study. Int J Clin Oncol 2011;16:57–62. [PubMed] DOI:10.1007/s10147-010-0135-9
- Iwasa S, Nakajima TE, Nakamura K, et al. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer 2012;15:21–6. [PubMed] DOI:10.1007/s10120-011-0056-y
- Xiao J, Chen Y, Li W, et al. Dose-dense biweekly docetaxel combined with 5-fluorouracil as first-line treatment in advanced gastric cancer: a phase II trial. Med Oncol 2015;32:334. [PubMed] DOI:10.1007/s12032-014-0334-8
- Hara T, Fujiwara Y, Sugimura K, et al. S-1 plus cisplatin combination therapy for gastric cancer with peritoneal dissemination. Gan To Kagaku Ryoho (in Japanese) 2015;42:1466–8. [PubMed]
- Ishiguro T, Fukuchi M, Ogura T, et al. A case of advanced gastric cancer successfully treated with curative conversion surgery after chemotherapy with S-1 plus oxaliplatin. Gan To Kagaku Ryoho (in Japanese) 2016;43:2213–15. [PubMed]
- Oneyama M, Sekikawa K, Goto M, et al. A long-surviving patient with unresectable advanced gastric cancer treated with S-1 and biweekly paclitaxel combination chemotherapy as second-line treatment. Gan To Kagaku Ryoho (in Japanese) 2012;39:1131–3. [PubMed]