Understanding the molecular pathogenesis and prognostics of bladder cancer: an overview
Introduction
The unparalleled developments in cancer genetics and genomics, achieved since the completion of the human genome project, are rapidly affecting clinical management and diagnostics in solid tumors. For patients with lung, colon, and breast cancer, molecular diagnostics is now an integral part of routine clinical management. However for current management algorithms of urologic malignancies these molecular biomarkers have not been widely applied (1,2).
Urinary bladder cancer (BC) is a heterogeneous disease with diverse morphologic and clinical manifestations (3). Global estimation suggests that in 2002, approximately 357,000 bladder cancer cases were diagnosed and about 145,000 patients died from this disease (4). This situation leads to the need for innovative treatment alternatives which can improve so-far the modest outcome in bladder cancer. However, well-validated prognostic molecular biomarkers that help clinicians to identify patients who need early aggressive management are currently lacking. Last but not the least, identifying robust predictive biomarkers which stratify response to emerging targeted therapeutics is another crucially needed development (2,5-7). This review will focuses on several promising candidate biomarkers which may soon make their transition to the realm of clinical management of bladder cancer.
FGFR3 and TP53 mutations define two key pathogenesis of urothelial carcinoma
Superficial and muscle-invasive urothelial carcinoma (UC) of the bladder display two distinct clinical phenotypes with regard to the biologic behavior and prognosis (Figure 1). In addition, molecular evidence supporting two divergent pathways of pathogenesis for superficial and invasive disease is accumulating. Superficial UC is thought to be originated from benign urothelium through hyperplasia with only a small contribution (10–15%) to the pool of high-grade noninvasive and subsequently invasive UC. Most invasive tumors appear to be originated through progression from dysplasia to flat carcinoma in situ (CIS) and high-grade noninvasive UC in which genetic instability leads to the accumulation of genetic alterations promoting progression to muscle-invasive bladder cancer (MI-BC) (1,8,9).
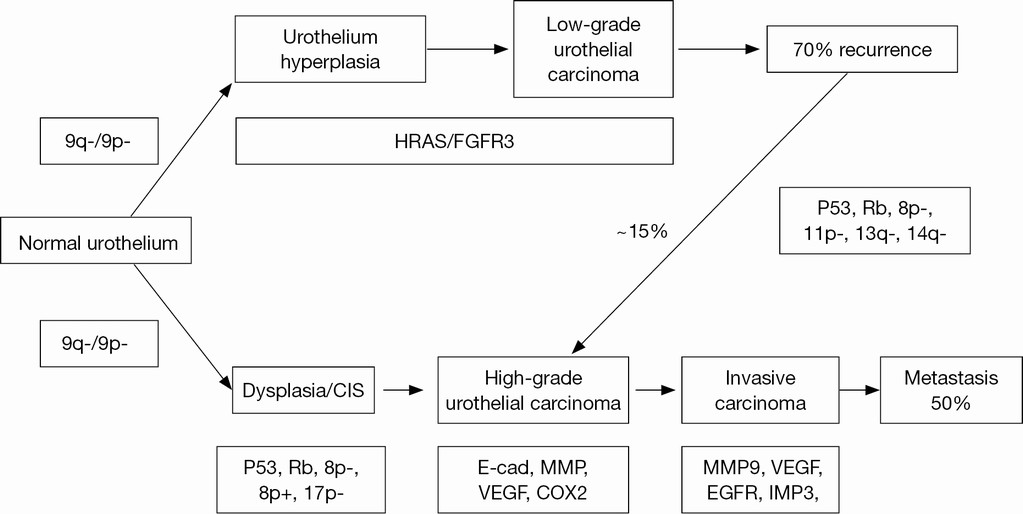
Clinically, a significant proportion of superficial tumors (pTa and pT1) will likely to recur following transurethral resection (TURB), but only a minority of cases will further progress to high-grade carcinoma and finally to MI-BC. Three primary genetic alterations have consistently been associated with the pathogenic pathway of superficial non-muscle-invasive bladder cancer (NMI-BC). These altered molecules include: Tyrosine kinase receptor, FGFR-3, H-RAS, and PI3KCA (10-13). Alterations in the RAS-MAPK and PI3K-Akt pathways are in mainly the cause for abnormal cell growth in urothelial neoplasia. Activating mutations in RAS lead to the activation of mitogen-activated protein kinase (MAPK) and PI3 K pathways. Not surprisingly, activating mutations in upstream tyrosine kinase receptor FGFR3 seem to be mutually exclusive with RAS mutations suggesting that both signals undergo a common downstream pathway in urothelial oncogenesis. PIK3CA and FGFR3 mutations are found generally co-occurred suggesting a potential synergistic additive oncogenic effect for PIK3CA mutations (9). The pathogenic pathway for MI-BC primarily involves alterations in tumor suppressor genes responsible for cell cycle control, including p53, p16, and Rb (14-16).
Prognostic biomarkers in superficial non–muscle invasive and muscle invasive urothelial carcinoma
Established clinicopathologic prognostic parameters for NMI-BC include pTNM stage, World Health Organization (WHO) /International Society of Urological Pathology grade, tumor size, tumor multifocality, presence of CIS, frequency and rate of prior recurrences (1). Prognostic parameters that can accurately predict progression in patients with superficial tumors are actively sought to further facilitate the identification of those in need of vigilant surveillance and aggressive treatment plan. Furthermore, given the current poor outcome of MI-BC (60% or less overall survival rate), markers that can improve prognostication in this group of patients are needed (17).
Numerous molecular factors are involved in determining UC phenotype, genotype, biological behavior, and clinical outcomes. Therefore, molecular technologies can be applied to these major carcinogenic alterations in searching for novel tumor markers (7). Molecular biomarkers are distinctive molecules produced by a tumor that are detectable and measurable in patient specimens and are representing various tumor properties. The most challenging task in molecular analysis of bladder cancer is to establish the clinical relevance of each molecular subgroup with respect to various tumor characteristics, beyond the histologic appearance. A variety of molecular markers, such as cell cycle regulators, cell proliferation promoters, signal transduction factors, apoptosis modulators, extracellular matrix-modulating molecules, and angiogenesis regulators, have been found to be associated with tumor grade and staging, risk of recurrence, and progression (1,5-7).
Numerical chromosomal alterations
Numerical chromosome aberrations represent changes of copy numbers of various genetic regions. Numerical chromosomal changes have been used widely for BC screening, diagnosis, and possibly prognostication. These aberrations can be detected by multicolor interphase fluorescence in situ hybridization (FISH), single nucleotide polymorphism analysis (SNP), or comparative genomic hybridization (CGH). The most frequent observed copy number aberrations in UC are on chromosomes 1, 8, 9, 10, 11, 13, and 14 (18). Chromosome 9 alterations are the earliest genetic alterations in both of the described divergent pathways of BC development. They are responsible for providing the necessary milieu of genetic instability that in turn allows for the accumulation of subsequent genetic defects. Several additional structural/numerical somatic chromosomal alterations also contributes to BC. Moreover, gains of chromosomes 3q, 7p, and 17q and 9p21 deletions (p16 locus) are of special interest which give them potential diagnostic and prognostic value (19).
Receptor tyrosine kinases
Recent studies have pointed out the potential prognostic value of evaluating the expression of receptor tyrosine kinases such as FGFR3, NRAS, HRAS, epidermal growth factor receptor (EGFR), and other ERB family members (HER2/neu and ERBB2) in superficial and muscle invasive BC disease (8,9,14). Previous studies have shown compelling evidence of frequent FGFR3 mutations in low grade bladder cancer (20). Later studies have shown that mutations in FGFR3 and lower expression of p53 are significantly correlated to lower risk of progression and higher disease specific survival (21). Recently, Kompier et al. (12) screened 253 primary and 184 recurrent bladder tumors using a mutational assay for detection of mutation in HRAS, KRAS and NRAS genes and combined this with assays for FGFR3 and PIK3CA oncogenes. In the pTa-T1 G1–2 groups, 88% of the primary tumors harbored a mutation in at least one of the five investigated oncogenes, mirroring their previous work that FGFR3 and PIK3CA mutations often co-occurred in this subset of tumors (22). In the high grade and muscle invasive tumor groups, the total percentage of mutations in the oncogenes was much lower with 33% and 36%, respectively. No individual mutation or combination of mutations was able to predict recurrence in the primary bladder tumors.
Even though no clear correlation of EGFR expression to outcome has been found consistently, ERBB2 over-expression has been found to be very common in high grade and invasive bladder cancers (5,7). Other studies have shown that although protein over-expression of her2/neu was very common and occurs in 70–90% of invasive tumors, gene amplification was seen only in 6–7% of these patients (23). Several studies have showed a prognostic significance of ERBB2 in assessing stage/grade and predicting cancer specific survival and metastases (24,25), but some have failed to find out any prognostic significance in multivariate analyses (26). In the most recent study of 198 patients, Bolenz and colleagues (25) found that patients having a her2/neu positive expression were twice as likely to recur and have cancer specific mortality compared to her2/neu negative patients independent of pathological tumor stage, grade, lymphatic vessel invasion, lymph node metastasis and adjuvant chemotherapy. Such studies merit further assessment on mechanistic and prognostic significance of ERBB2 in BC.
P53, cell cycle regulators, and proliferation index markers
Altered cell cycle control is a hallmark of BC driven by both aberrant signal transduction as well as key alterations in cell cycle molecules such as p53 and pRb (5). Mutations in TP53 which codes for the p53 tumor suppressor protein, is critical in BC. Missense mutations in TP53 lead to an altered protein that is resistant to degradation through the ubiquitin pathway and results in nuclear accumulation of p53. This allows dysregulating progression of the cell through the G1-S checkpoint and drives cancer development and progression through altered apoptosis, DNA repair and response to therapy (27). Early studies have showed that p53 expression is strongly correlated with stage, progression and mortality of BC (28). However, one recently large multicenter study has suggested that p53 alone has minimal prognostic benefit over clinicopathological models (29).
Among other G1-S phase cell cycle regulators, cyclinsD3 and D1, p16, p21, and p27 have also been evaluated as prognosticators in NMI-BC (1). Lopez-Beltran et al confirmed their initial finding of the independent prognostic role of cyclin D3 and cyclin D1 overexpression in predicting progression in pTa and pT1 tumors (30,31). Their findings, however are in contrast to subsequent findings by Shariat et al. (32), thereby emphasizing the need for further validation in multi-institutional large cohorts of patients. A synergistic prognostic role for combining p53 evaluation with other cell cycle control elements such as pRb, cyclin E1, p21, and p27 is emerging in both NMI-BC and MI-BC (6). Shariat and colleagues (33) found that the number of such markers altered, often corresponding to disease severity.
More recently, Mitra and colleagues (34) showed that a nine panel marker comprising Bax, caspase-3, apoptotic protease activating factor 1 (Apaf-1), Bcl-2, p53, p21, cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), and E-cadherin and smoking intensity, these molecules had significantly greater accuracy in a multivariate model in predicting survival in 212 bladder cancer patients than routine clinicopathological factors alone, despite none of the individual markers being significant. The number of altered markers was also significantly linked to survival rate. Such panels now worth further prospectively validation to assess their efficacy and cost effectiveness in bladder cancer management.
The proliferation marker Ki-67 has also been shown in studies to independently predict recurrence and progression free survival of NMI-BC as well as progression after cystectomy for MI-BC (35,36). In the study by Quintero et al. (35), Ki-67 index in NMI-BC TURB biopsy samples was predictive of progression free survival. A similar role for proliferation index assessment as a prognosticator is established in MI-BC. Based on the initial findings of significance in an organ-confined subset of MIBC by Margulis et al. (36), a recent report of the bladder consortium multi-institutional trial (7 institutions; 713 patients) again verified the role of proliferation index using cystectomy specimens. In one later study (37), Ki-67 improved the prediction of both progression free survival and disease-specific survival when added to standard prediction models, suggesting a role for proliferation index assessment in stratifying patients for perioperative systemic chemotherapy. These studies have certainly taken Ki-67 assessment a step closer to its clinical applicability in MI-BC.
Gene expression and genomic analysis
Global approaches as well as pathway specific approaches continue to provide an insight into the pathways involved in development and progression of BC (7). Several studies have used these approaches to identify presence or absence of disease, validate different pathways involved in different stages and grades of BC and finally to predict prognosis in NMI-BC and MI-BC (6). In a landmark study by Sanchez-Carbayo et al. (38) oligonucleotide arrays were used to analyze transcript profiles of 105 cases of NMI-BC and MI-BC. Hierarchical clustering and supervised algorithms were used to stratify bladder tumors by stage, nodal metastases, and overall survival. Predictive algorithms were 89% accuracy for tumor staging using genes differentially expressed in superficial versus muscle invasive tumors. Accuracies of 82% and 90% (MI-BC) were also obtained for predicting overall survival. A genetic profile consisting of 174 probes was showed to be able to identify patients with positive lymph nodes and poor survival (38). In a recent study, Lindgren et al. (39) suggested that a combined molecular and histopathologic classification of BC may prove more powerful in predicting outcome and stratifying treatment. The authors combined the gene expression analysis, whole-genome array comparative genomic hybridization analysis, and mutational analysis of FGFR3, PIK3CA, KRAS, HRAS, NRAS, TP53, CDKN2A, and TSC1 to identify 2 intrinsic molecular signatures (MS1 and MS2). Genomic instability was the most genomic feature of MS2 signature, independent of TP53/MDM2 alterations. Their genetic signatures were validated in 2 independent data sets that successfully classified urothelial carcinomas into low-grade and high-grade tumors, as well as NMI-BC and MI-BC, and are with high precision and sensitivity. Furthermore, a gene expression signature that independently predicts metastasis and disease free survival was also defined. For prediction of recurrence of MI-BC after cystectomy, a most recently study by Mitra et al. (40) showed that genomic-based classifiers outperformed clinical models for predicting postcystectomy bladder cancer recurrence, and this may be used to better identify patients who need more aggressive management. Above analysis clearly supports the role of molecular grading as a complement to standard pathologic grading.
Probably the most comprehensive molecular subclassification study has been performed by The Cancer Genome Atlas (TCGA) project (41). In this landmark study that examined 131 cases of MI-BC, the investigators examined data on whole-genome sequencing, whole-exome sequencing, DNA copy number, complete mRNA and microRNA expression, DNA methylation, and protein expression and phosphorylation. They found consistent mutations in many genes previously identified, including TP53, PIK3CA, RB1, FGFR3, and TSC1, confirming many prior studies. In addition, they were able to subclassify muscle-invasive urothelial carcinoma into 4 different molecular types based on expression of specific mRNAs and proteins. Integrating all of the data, they were able to identify a few pathways that are consistently dysregulated in BC, including the p53/RB tumor suppressor pathway and the PI3K/AKT/mTOR and the RTK/RAS pathways, that affect cell proliferation and survival and pathways that affect epigenetic changes, such as chromatin remodeling and histone modification. These latter pathways, which affect epigenetic pathways, were seen in 89% of bladder tumors, more than in any other cancer studied, suggesting that there may be many subtle epigenetic causes of urothelial neoplasia that are still poorly understood (41).
Epigenetic alterations
Epigenetic analysis is also gaining momentum in BC as a noninvasive diagnostic tool for screening and surveillance. As a prognostic tool, epigenetic analysis has shown promising potential in patients with BC (42). Aberrant DNA methylation and histone modification have been proved to play a role in regulating gene expression and may contribute to carcinogenesis (7). Multiple groups have demonstrated that hypermethylation of RARB, RASSF1 and DAPK is linked to aggressiveness of BC (1). Catto et al. (43) found that in a large cohort of 280 upper and lower tract BCs, promoter methylation was more common in upper tract tumors (94%) than lower ones (76%) and was significantly associated with tumor stage, progression and mortality. RASSF1 and DAPK1 hypermethylation was correlated with tumor progression independent of clinicopathological risk factors on multivariate analysis. The same group later have additionally showed that a panel of RASSF1a, E-cadherin, TNFSR25, EDNRB, and APC hypermethylation was associated with tumor stage, progression and death (44). Epigenetic predictive models validated the presence and timing of tumor progression with 97% specificity and 75% sensitivity. Agundez et al. (45) profiled the methylation status of 25 tumor suppressor genes in 91 T1G3 tumors treated with bacillus Calmette-Guerin (BCG) using a methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) assay. Univariate analysis demonstrated that methylation of PAX6 was associated with tumor recurrence and methylation of several genes and was associated with progression. Additionally, multivariate analysis have showed that the gene MSH6 and THBS-1can serve as predictors for tumor progression (45). This was a promising finding in a clinical situation in which prognostication is critical. If validated independently and shown to add power to the current clinical algorithms, such biomarkers could help identify high risk patients for early cystectomy. Above reviewed studies now should consist of a panel of multiple markers, ideally be multi-institutional, and have robust study design in a specific clinical setting and should be sufficiently powered to draw conclusions about whether the panel would provide additional benefit over current clinicopathological models.
Conclusions
In summary, as our understanding of the complex molecular mechanisms involved in the BC development has came into a deeper focus, our approaches for diagnosis and management of BC is also progress. In the future, the paradigm of clinicopathologic based prognostic approach for the prediction of superficial BC progression will be supported by the molecular-guided approach including several markers mentioned above. Several new targeted therapy agents are now under investigation in combination with standard chemotherapy agents in randomized trials. These promising agents later might be served as first-line treatment or as a maintenance basis to prolong the therapy response in patients with advanced BC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Netto GJ, Cheng L. Emerging critical role of molecular testing in diagnostic genitourinary pathology. Arch Pathol Lab Med 2012;136:372-90. [PubMed]
- Xylinas E, Kluth LA, Rieken M, et al. Urine markers for detection and surveillance of bladder cancer. Urol Oncol 2014;32:222-9. [PubMed]
- Bostwick DG, Cheng L. Urologic Surgical Pathology. 3rd ed. Philadelphia, PA: Elsevier/Saunders, 2014.
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Cheng L, Davison DD, Adams J, et al. Biomarkers in bladder cancer: translational and clinical implications. Crit Rev Oncol Hematol 2014;89:73-111. [PubMed]
- Sapre N, Herle P, Anderson PD, et al. Molecular biomarkers for predicting outcomes in urothelial carcinoma of the bladder. Pathology 2014;46:274-82. [PubMed]
- Cheng L, Zhang S, MacLennan GT, et al. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol 2011;42:455-81. [PubMed]
- Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol 2009;4:251-85. [PubMed]
- Al Hussain TO, Akhtar M. Molecular basis of urinary bladder cancer. Adv Anat Pathol 2013;20:53-60. [PubMed]
- Crawford JM. The origins of bladder cancer. Lab Invest 2008;88:686-93. [PubMed]
- Cheng L, Davidson DD, Maclennan GT, et al. The origins of urothelial carcinoma. Expert Rev Anticancer Ther 2010;10:865-80. [PubMed]
- Kompier LC, Lurkin I, van der Aa MN, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One 2010;5:e13821. [PubMed]
- Millis SZ, Bryant D, Basu G, et al. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer 2015;13:e37-49. [PubMed]
- Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer 2005;5:713-25. [PubMed]
- Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552-64. [PubMed]
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25-41. [PubMed]
- Shariat SF, Chade DC, Karakiewicz PI, et al. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol 2010;183:68-75. [PubMed]
- Matsuyama H, Ikemoto K, Eguchi S, et al. Copy number aberrations using multicolour fluorescence in situ hybridization (FISH) for prognostication in non-muscle-invasive bladder cancer (NIMBC). BJU Int 2014;113:662-7. [PubMed]
- Kawauchi S, Sakai H, Ikemoto K, et al. 9p21 index as estimated by dual-color fluorescence in situ hybridization is useful to predict urothelial carcinoma recurrence in bladder washing cytology. Hum Pathol 2009;40:1783-9. [PubMed]
- Pandith AA, Shah ZA, Siddiqi MA. Oncogenic role of fibroblast growth factor receptor 3 in tumorigenesis of urinary bladder cancer. Urol Oncol 2013;31:398-406. [PubMed]
- Hernández S, López-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol 2006;24:3664-71. [PubMed]
- López-Knowles E, Hernández S, Malats N, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res 2006;66:7401-4. [PubMed]
- Latif Z, Watters AD, Dunn I, et al. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer 2004;40:56-63. [PubMed]
- Eissa S, Ali HS, Al Tonsi AH, et al. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin Biochem 2005;38:142-8. [PubMed]
- Bolenz C, Shariat SF, Karakiewicz PI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int 2010;106:1216-22. [PubMed]
- Gandour-Edwards R, Lara PN Jr, Folkins AK, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 2002;95:1009-15. [PubMed]
- Petitjean A, Achatz MI, Borresen-Dale AL, et al. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 2007;26:2157-65. [PubMed]
- Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 2005;6:678-86. [PubMed]
- Shariat SF, Bolenz C, Karakiewicz PI, et al. p53 expression in patients with advanced urothelial cancer of the urinary bladder. BJU Int 2010;105:489-95. [PubMed]
- Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in stage T1 grade 3 bladder cancer survival: the role of G1-S modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1). Eur Urol 2004;45:606-12. [PubMed]
- Lopez-Beltran A, Requena MJ, Luque RJ, et al. Cyclin D3 expression in primary Ta/T1 bladder cancer. J Pathol 2006;209:106-13. [PubMed]
- Shariat SF, Ashfaq R, Sagalowsky AI, et al. Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol Oncol 2007;25:468-75. [PubMed]
- Shariat SF, Zlotta AR, Ashfaq R, et al. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol 2007;20:445-59. [PubMed]
- Mitra AP, Castelao JE, Hawes D, et al. Combination of molecular alterations and smoking intensity predicts bladder cancer outcome: a report from the Los Angeles Cancer Surveillance Program. Cancer 2013;119:756-65. [PubMed]
- Quintero A, Alvarez-Kindelan J, Luque RJ, et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol 2006;59:83-8. [PubMed]
- Margulis V, Shariat SF, Ashfaq R, et al. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res 2006;12:7369-73. [PubMed]
- Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst 2009;101:114-9. [PubMed]
- Sanchez-Carbayo M, Socci ND, Lozano J, et al. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol 2006;24:778-89. [PubMed]
- Lindgren D, Frigyesi A, Gudjonsson S, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 2010;70:3463-72. [PubMed]
- Mitra AP, Lam LL, Ghadessi M, et al. Discovery and validation of novel expression signature for postcystectomy recurrence in high-risk bladder cancer. J Natl Cancer Inst 2014.106. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [PubMed]
- Abern MR, Owusu R, Inman BA. Clinical performance and utility of a DNA methylation urine test for bladder cancer. Urol Oncol 2014;32:51.e21-6. [PubMed]
- Catto JW, Azzouzi AR, Rehman I, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol 2005;23:2903-10. [PubMed]
- Yates DR, Rehman I, Abbod MF, et al. Promoter hypermethylation identifies progression risk in bladder cancer. Clin Cancer Res 2007;13:2046-53. [PubMed]
- Agundez M, Grau L, Palou J, et al. Evaluation of the methylation status of tumour suppressor genes for predicting bacillus Calmette-Guérin response in patients with T1G3 high-risk bladder tumours. Eur Urol 2011;60:131-40. [PubMed]
