Postoperative chemotherapy with S-1 plus oxaliplatin versus S-1 alone in locally advanced gastric cancer (RESCUE-GC study): a protocol for a phase III randomized controlled trial
Background and rationale
Gastric cancer is the third most common cause of cancer-related death worldwide (1). D2 gastrectomy is the standard of care in Asia (2,3). Various regimens for adjuvant chemotherapy have been recommended for the resectable gastric cancer to prevent postoperative recurrence (4-6). Two large randomized controlled trials, ACTS-GC and CLASSIC, have investigated the efficacy of adjuvant chemotherapy after D2 gastrectomy vs. D2 gastrectomy alone for patients with resectable gastric cancer. The ACTS-GC trial reported patients with stage II or IIIA gastric cancer who received S-1 after curative gastrectomy had better outcomes compared with patients who received surgery only (7). The 5-year overall survival (OS) rate was 71.7% in the S-1 group compared with 61.1% in the surgery only group. In the CLASSIC trial, the survival benefit was consistent with that in ACTS-GC (8). The 5-year OS rate was improved in the adjuvant capecitabine plus oxaliplatin group (78% vs. 69% in the surgery-only group). The benefits of fluoropyrimidine-based chemotherapy with one agent or two agents in stage II and IIIA gastric cancer were both considerable.
A meta-analysis suggests that treatment with several drugs could be more effective than treatment with using fewer drugs (9), but this finding has not been confirmed in the adjuvant setting (10). None of the sequential S-1-paclitaxel, sequential tegafur and uracil (UFT)-paclitaxel resulted in a superior outcome when compared with single-agent, adjuvant S-1 in the trial SAMIT (11). Nevertheless, no clinical trial evaluated the efficacy of adjuvant chemotherapy with S-1 plus oxaliplatin when compared to adjuvant S-1 alone. Because of high incidence of locally advanced gastric cancer in China, increasing number of older patients with comorbidities, conflicts between costly treatment strategy and limited medical resources, an effective, economical and feasible regimen for adjuvant chemotherapy is crucial there. In the current trial, we hypothesize that efficacy of adjuvant chemotherapy with S-1 in resectable gastric cancer is not inferior to that of S-1 plus oxaliplatin. Patients with stage II and IIIA gastric cancer are eligible. Postoperative chemotherapy with S-1 will be explored in a randomized comparison with S-1 plus oxaliplatin, one of the standard treatment options.
Digest of study protocol
Objectives
The aim of this trial is to evaluate the efficacy of S-1 alone compared with S-1 plus oxaliplatin for adjuvant treatment in locally advanced gastric cancer after D2 gastrectomy.
Study setting
This trial is a multi-institutional, prospective, open-label, randomized phase III trial.
Endpoints
The primary endpoint is the 3-year relapse-free survival (RFS) of all randomized patients. RFS is counted from the date of registration to the earliest date of tumor recurrence, and it is censored at the latest day when the patient is confirmed to be alive without any evidence of recurrence. The diagnosis of tumor relapse should be based on objective evidence of imaging test [computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-computed tomography (PET-CT), B-ultrasound], endoscopy and/or histology. Elevated serum tumor marker cannot be used as direct evidence of tumor relapse. The secondary endpoints are 5-year OS, proportion of patients who complete the protocol treatment, and incidence of adverse events.
Eligibility criteria
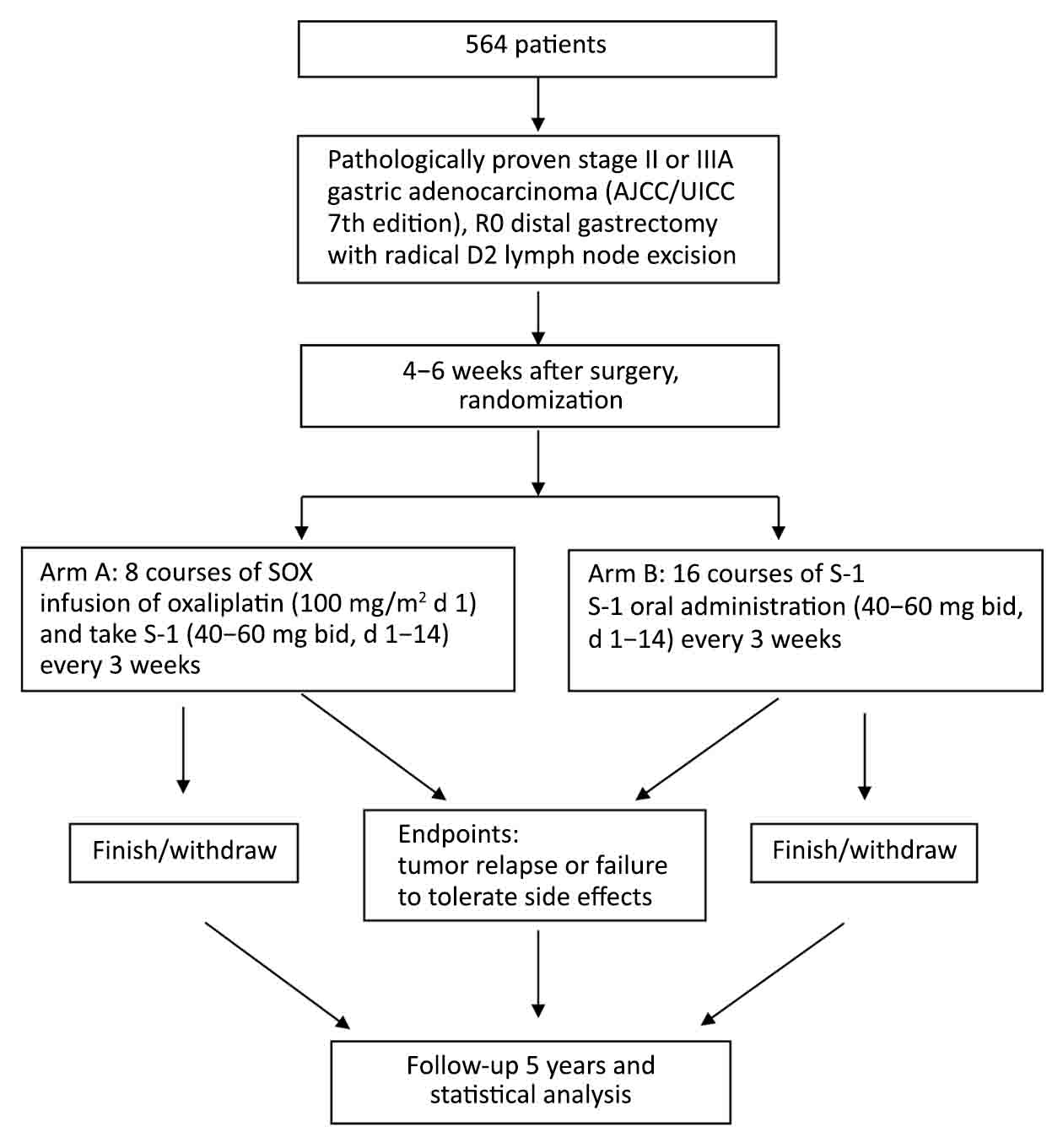
Prior to enrollment in this trial, the patients must meet all the criteria below: 1) pathologically proven stage II or IIIA gastric adenocarcinoma. (The 8th edition of AJCC TNM classification); 2) no hepatic, peritoneal or distant metastasis; 3) negative peritoneal cytology; 4) R0 distal gastrectomy with radical D2 lymph node excision; 5) aged 18–70 years old; 6) performance status of either 0 or 1 on Eastern Cooperative Oncology Group (ECOG) criteria; 7) no previous chemotherapy or radiotherapy; 8) adequate major organ function as evidenced by the following laboratory findings: WBC >4.0×109/L, PLT >100×109/L, ALT and AST ≤2.5 times the upper limit of normal, and total bilirubin ≤1.5 times the upper limit of normal; 9) no unstable cardiac dysfunction and other organ diseases; 10) able to start chemotherapy 4–6 weeks after surgery; 11) not enrolled in other trials; and 12) written informed consent.
Exclusion criteria
Prior to enrollment in this trial, the patients must not meet any of the criteria below: 1) pregnant, possibly pregnant or lactating; 2) underwent prior treatment such as chemotherapy and radiotherapy; 3) history of serious drug hypersensitivity; 4) infectious disease requiring systemic therapy; 5) serious concomitant disease; or 6) determined by the investigator to be unsuitable for other reasons.
Randomization and allocation concealment
After confirming fulfillment of the eligibility criteria, registration is made by a web-based system to the Data Center (https://crabyter.sinyoo.net/). Patients will be randomized to either arm A [adjuvant chemotherapy with S-1 plus oxaliplatin (SOX)] or arm B (adjuvant chemotherapy with S-1 only). The minimization method will be used for the randomization of patients, thereby balancing the arms of the study according to gender, tumor depth and the institution. We hypothesize that both 3-year RFS and 5-year OS of arm B are not inferior to those of arm A. If no significant difference in patient survival of the two arms, the regimen with S-1 will be concluded as the standard treatment for II and IIIA stage gastric cancer after radical surgery.
Treatment methods
Postoperative chemotherapy
After the R0 resection, adjuvant chemotherapy will be initiated within 4–6 weeks from surgery (Figure 1). Patients in the arm A will receive 8 courses of SOX including an infusion of oxaliplatin (100 mg/m2 d 1) and take S-1 (40–60 mg bid, d 1–14) every 3 weeks. Patients in the arm B will receive 16 courses of S-1 oral administration (40–60 mg bid, d 1–14) every 3 weeks. If the adjuvant treatment is not initiated within 6 weeks after surgery for any reason, the protocol treatment is terminated. The protocol treatment is terminated and any treatment is allowed when tumor relapse.
Adverse events and safety monitoring plan
An independent data and safety monitoring board (DSMB) will be formed to monitor data and safety. Incidence of adverse events will be graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Severe adverse events resulting from the trial will be informed to the principle investigator and be treated until symptoms typically disappear or become less intense.
Follow-up
Patients will be followed-up every 3 months for the following 2 years after surgery, and subsequently every half year for the next 3 years. Follow-up evaluations will include a physical examination, blood tests such as complete blood cell count, measurements of liver and renal function, gastrointestinal tumor markers, abdominal and pelvic enhanced CT scan, chest plain CT scan and hepatic enhanced MRI scan when atypical focal liver lesion occurs. Upper gastrointestinal endoscopy will be conducted per year.
Sample size calculation and statistical analysis
According to the statistical tools on SWOG center (http://www.swogstat.org/), the required sample size will be 564 patients (282 patients per arm), with a one-sided alpha level of 5% and a power of 80%. Analyses of efficacy of chemotherapy were based on both intent-to-treat (ITT) set and the per-protocol (PP) set. Data related to patient characteristics will be compared between two arms by using the Chi-square test. Cumulative survival will be estimated with the Kaplan-Meier method, and comparisons between the arms will be done with a Log-rank test. A multivariate analysis of the Cox proportional hazards regression model (backward, stepwise) will be created to assess the influence of each variable on survival. Significance will be set at P<0.05. All statistical analyses were carried out using the IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA).
Clinical trials registry
This trial has been registered in the ClinicalTrials.gov as NCT02867839 (https://clinicaltrials.gov/).
Participating institutions
Peking University Cancer Hospital & Institute, the First Affiliated Hospital of Dalian Medical University, Chinese People’s Liberation Army General Hospital, Zhejiang Cancer Hospital, Peking University First Hospital, Peking University People’s Hospital, Beijing Hospital, Beijing Friendship Hospital of Capital Medical University, Xuanwu Hospital of Capital Medical University, Qilu Hospital of Shandong University, the First Affiliated Hospital of Wenzhou Medical University, Nanjing General Hospital, and Zhongnan Hospital of Wuhan University.
Trial status
The trial has been approved by the institutional review board of each participating institution and it was activated on December, 2016. The enrollment will be finished in December, 2018. Patient’s follow-up will be ended until December, 2023.
Acknowledgements
This study is supported by the National Science Foundation of China (No. 81374016 and 81402308), Beijing Municipal Science & Technology Commission (No. D141100000414002).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [PubMed] DOI:10.3322/caac.21262
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [PubMed] DOI:10.1007/s10120-016-0622-4
- Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87–104. [PubMed] DOI:10.5230/jgc.2014.14.2.87
- Nakajima T, Kinoshita T, Nashimoto A, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg 2007;94:1468–76. [PubMed] DOI:10.1002/bjs.5996
- Shitara K, Chin K, Yoshikawa T, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer 2017;20:175–81. [PubMed] DOI:10.1007/s10120-015-0581-1
- Takahari D, Hamaguchi T, Yoshimura K, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol 2011;67:1423–8. [PubMed] DOI:10.1007/s00280-010-1432-8
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011; 29 :4387–93. [PubMed] DOI:10.1200/JCO.2011.36.5908
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389–96. [PubMed] DOI:10.1016/S1470-2045(14)70473-5
- Wagner AD, Grothe W, Haerting J, et al. Chemo-therapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–9. [PubMed] DOI:10.1200/JCO.2005.05.0245
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, et al. Benefit of adjuvant chemo-therapy for resectable gastric cancer: a meta-analysis. JAMA 2010;303:1729–37. [PubMed] DOI:10.1001/jama.2010.534
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886–93. [PubMed] DOI:10.1016/S1470-2045(14)70025-7