Germline mutations in hereditary diffuse gastric cancer
Introduction
Gastric cancer is one of the leading causes of cancer-related deaths worldwide. The vast majority of gastric cancers are sporadic. However, approximately 1%–3% of gastric cancers were characterized by inherited gastric cancer predisposition syndromes, knowing as hereditary diffuse gastric cancer (HDGC) (1). Patients diagnosed as HDGC normally have poor prognosis. HDGC is a poorly differentiated adenocarcinoma, especially signet ring carcinoma, mucinous adenocarcinoma and isolated cell-type carcinoma that infiltrates into the stomach wall causing thickening of the wall (linitis plastica) without forming a distinct mass. The average onset age of HDGC is 38 years (2).
The diagnostic standards for HDGC was established by the International Gastric Cancer Linkage Consortium (IGCLC) in 1999 (3), which suggested that individuals meet any of the following criteria could be diagnosed as HDGC: “Two or more cases of diffuse gastric cancer (DGC) in first- or second-degree relatives with at least one diagnosed prior the age of 50”; or “Three or more cases of DGC in first- or second-degree relatives, regardless of age of onset”. With the development of understanding of HDGC, these criteria were updated by adding two more criteria in 2010 by IGCLC (4), taking individuals with early onset age and lobular breast cancer (LBC) into consideration. In addition, the updated four criteria were also recommended as standards for genetic testing and genetic counseling by National Comprehensive Cancer Network (NCCN) guideline and the American College of Medical Genetics and Genomics (ACMG) as well as the National Society of Genetic Counselors (NSGC) (5), respectively. For the purpose of improving the performance of diagnosis for HDGC, the first two criteria were merged into one in 2015, resulting in the relaxation of restriction on age limit by IGCLC (6). To date, the newly updated criteria are still recommended for diagnosis of HDGC and as standards of sequencing in CDH1 germline mutations. Therefore, the current well-recognized standards for diagnosis of HDGC and genetic testing for CDH1 germline mutation consist of the following three criteria: “Two gastric cancer cases in first or second relatives, independent of age, at least one confirmed DGC”; or “Sporadic DGC prior to age 40”; or “Individuals and families with both DGC and LBC either of which is diagnosed prior to the age of 50 years”.
Genetically, HDGC is a rare autosomal dominant inherited gastric cancer syndrome. It has been reported that 25%–30% of families who fulfilled criteria for HDGC were caused by germline alterations in CDH1 gene (4). In addition, vast majority of individuals inherited a pathogenic variant predisposing to DGC from one parent. Each child of a proband bears a 50% risk of inheriting the cancer-predisposing variant. Moreover, CDH1 mutation in young individuals with DGC could be potentially from a family with no history of DGC, suggesting the importance of genetic testing for CDH1 in such population (7). However, in eastern Asian countries, where gastric cancer incidence is relatively high (8), the detection rates for germline CDH1 mutations are low. Therefore, screenings and genetic testing for HDGC were previously considered not of importance. HDGC is characterized by signet ring carcinoma and considered to be a type of cancer with poor prognosis. Mostly linked with CDH1 germline mutations, HDGC holds high mortality, whereas LBC is the second most frequent type of carcinoma (9). It is illustrated by Dossus et al. that CDH1 is associated with invasive lobular carcinoma (ILC) (10). The first paper demonstrating the association between breast cancer risk and HDGC was published in 2000 (11,12). In some researchers’ view, it is of importance to recognize that HDGC is a syndrome and that LBC can be the first manifestation of this syndrome (13). Therefore, early-onset of LBC could potentially warrant one that considers diagnosis of HDGC.
CDH1 (encoding the cell to cell adhesion protein E-cadherin) germline mutations was found in 30%–50% HDGC patients and the cumulative risk of CDH1 germline mutation carriers developing gastric cancer by the age of 80 years is approximately 70% for men and 60% for women (14). In addition, female carriers also hold a 39%–52% risk of breast cancer (14), and the association between loss of E-cadherin and ILC was reported by Christgen et al. (15). Germline mutations appear to be rare in the countries with high morbidity of sporadic gastric cancer. However, the reason remains unknown. Moreover, CDH1 Germline mutations have been identified in different ethnic groups (7,16-19). It was postulated that the different genetic background from various ethnicities may have different effects on the viability of embryos that already have one mutated germline CDH1 allele (20,21). CDH1 germline alterations encompass small frameshifts, splice-site, nonsense and missense mutations, as well as large rearrangements. Most CDH1 truncating mutations are pathogenic, and several missense CDH1 mutations have deleterious effect on E-cadherin function. Truncating alleles of CDH1 were firstly identified in Maori (indigenous New Zealander) families in which 25 members had died from DGC at young ages, typically at the age of 14–40 years (22). Ongoing studies focused on the assessment of the pathogenicity and penetrance of CDH1 germline mutations, however, for the majority of HDGC families, the genetic cause remains unknown (23). The investigations of genetic causes other than CDH1 germline defects were focused on those with strong HDGC family history but without CDH1 mutations. Recently, germline mutations of some related genes, such as CTNNA1, MAP3K6, INSR, FBXO24, DOT1L MAP3K6, CD44, PALB2, BRCA1, RAD51C and MET, have been found to be susceptible for special HDGC family respectively (24,25).
In this review, we briefly introduced the development of the studies seeking new germline mutations in HDGC, and the new techniques and strategies which spring up before a focused discussion based on most recent data.
Germline mutations in HDGC
CDH1
CDH1 is a tumor suppressor gene located on chromosome 16q22, comprising 16 exons that span 100 kb and encoding E-cadherin protein. E-cadherin is a transmembrane glycoprotein responsible for physical connection of epithelial cells through Ca(2+)-binding regions in its extracellular domain, exerting cell-cell adhesion and invasion-suppression functions (26). E-cadherin is critical for the establishment and maintenance of polarized and differentiated epithelia during development (27). It also plays important roles in signal transduction, rearrangement of cells and tissue morphogenesis (28). The activity of E-cadherin in cell adhesion depends on its association with the actin cytoskeleton via undercoat proteins called catenins (α-, β- and γ-) (29,30).
From the first report (22) to date, more than 100 germline mutations of CDH1 have been reported in families with HDGC (3,26,31-35). The mutations are primarily truncating mutations, usually through frameshift mutations, exon/intron splice site mutations, or single nucleotide variants (3,22,36-39). Moreover, large exonic deletions make up of approximately 4% of these mutations (20). In general, no “hot spots” have been identified and the pathogenic variants have been found distributed throughout the entire gene. Additionally, there are reports on the same pathogenic variant found in several unrelated families, such as c.1003C>T in exon 7 (18,23,31), 1137G>A splicing mutation in exon 8 (18,32,40), c.1901C>T in exon 12 (18,32,41) and a founder mutation 2398delC in four families from Newfoundland, Canada (18). In general, truncating mutations are assumed to be pathogenic, however, clinical management in individuals with missense mutations remains to be elucidated, therefore, both extensive family data and functional data are required for the prediction of pathogenicity caused by a missense mutation (3,38,42). In the absence of such data, it may not be appropriate to use CDH1 missense mutation to define risks. The pathogenicity of missense mutations can be investigated through in vitro analysis, although this is only performed on a research basis (43). For instance, c.1018A>G in CDH1, a known disease-causing mutation, was found in a Korean case of pre-symptomatic detection of CDH1 mutation (44).
CTNNA1
E-cadherin-mediated cell-cell adhesion is affected by 3 cytoplasmic proteins known as α-catenin, β-catenin and γ-catenin. They are identified to work as connectors that anchor E-cadherin to the cytoskeletal actin bundle through cadherin cytoplasmic domain (26). E-cadherin/catenin complex is a powerful inhibitor of invasion. Dysfunction of this adhesion complex causes dissociation of cancer cells from primary tumor nodules, thus possibly contributing to cancer invasion and metastasis (45). As a binding partner of E-cadherin, mutated β-catenin and γ-catenin have been considered as candidates for DGC predisposition (46). Genetic variations of CTNNA1 have been found to be associated with clinical pathological features. Colorectal cancer (CRC) patients with CTNNA1 mutation exhibited significantly increased lymph node metastasis (47).
Majewski et al. identified a 2 bp germline deletion in exon 2 of CTNNA1, which results in a frameshift after Arg27 (p.Arg27Thr.fs*17) using exome sequencing, mass spectrometry genotyping and candidate gene resequencing in a large Dutch HDGC pedigree with no obvious mutation in CDH1 (24). However, CTNNA1 protein expression was found lost in tumors from this family. In detail, CTNNA1 germline truncating allele was presented in two family members with invasive DGC and four in which intramucosal signet ring cells were detected as part of endoscopic surveillance. The remaining CTNNA1 allele was silenced in the two DGCs from the family that were available for screening, and this was also true for signet ring cells identified in endoscopic biopsies. This suggests that CTNNA1 may have the potential to be associated with invasiveness of DGC. However, tests in other family pedigrees which fulfilled clinical criteria of HDGC showed that loss of CTNNA1 was only found in one of ten tumors in CDH1 wild-type HDGC biopsies (24) . Therefore, evidence from functional studies of in vitro and in vivo DGC models is required to identify the association between CTNNA1 and DGC. This was the first report for germline mutation other than CDH1 in HDGC. Since CTNNA1 functions in the same complex as E-cadherin, their results called attention to the broader signaling network surrounding these proteins in HDGC (24).
Other potential candidate susceptibility genes
CDH1 germline mutations were identified in HDGC widely in the European and American countries, with the incidence of greater than 25% (4,14), however, the diagnostic rate in Eastern countries, such as China, was much more lower (data not published). Such discrepancy may attribute to diverse ethnic groups from different regions. Moreover, studies on seeking potential CTNNA1 germline mutations in HDGC patients without CDH1 mutation exhibited contradicted results, suggesting that more HDGC family pedigrees may be required or functional studies are needed to further elucidate the association between HDGC and lost function of catenin caused by CTNNA1 germline mutation. For the uncertainty of pathogenesis in many gastric cancer families linked to cancer predisposition syndromes without CDH1 germline mutations, it is necessary to continue exploring the potential candidate susceptibility genes of HDGC. Donner et al. identified the variants of INSR, FBXO24 and DOT1L as new candidates of DGC susceptibility genes in a Finnish HDGC pedigree (25). INSR has been shown to affect tumor cell invasion by modulating E-cadherin glycosylation, proposing its potential predisposition in HDGC. Studies indicated that FBXO24 may lead to malignancies and DOT1L has influences on DNA repair, therefore, they may act as new susceptibility genes contributing to HDGC. However, further studies are required to investigate whether there is association between FBXO24 and DOT1L mutations in HDGC (1). In addition, MAP3K6 is also a newly implicated gene which is associated with gastric cancer and acts as a tumor suppressor gene (1). Gaston et al. reported that MAP3K6 mutation is related to familial gastric cancer (48). CD44 is a cell surface receptor for hyaluronate, which encodes several protein isoforms. It is reported by da Cunha et al. that CD44 increasingly expressed in malignant lesions including HDGC, and overexpressed when E-cadherin was absent. Therefore, CD44 was suggested to be a predictable marker for HDGC patients not only carrying CDH1 mutations but loss of E-cadherin expression (49). Mutations of some genes, such as PALB2, BRCA1 and RAD51C, regulating homologous DNA recombination, were detected in HDGC patients with a proportion of 6.5%, whereas mutations in these genes were only found in 2.8% of sporadic gastric cancer patients, reported by Sahasrabudhe et al. (50). MET gene encodes a protein with an extracellular, transmembrane and a tyrosine kinase domain. The mutation of this gene, previously being reported in patients with hereditary papillary renal carcinoma, was firstly found by Kim et al. in a Korean patient with familial gastric cancer (51).
Although new germline mutations are rare and the sample size of some existed studies are too small to draw any conclusions, they warranted further studies to investigate the association of HDGC and other potential gene candidates in addition to CDH1 in the cohorts of CDH1 mutation-negative hereditary or familial diffuse gastric cancer (Table 1).
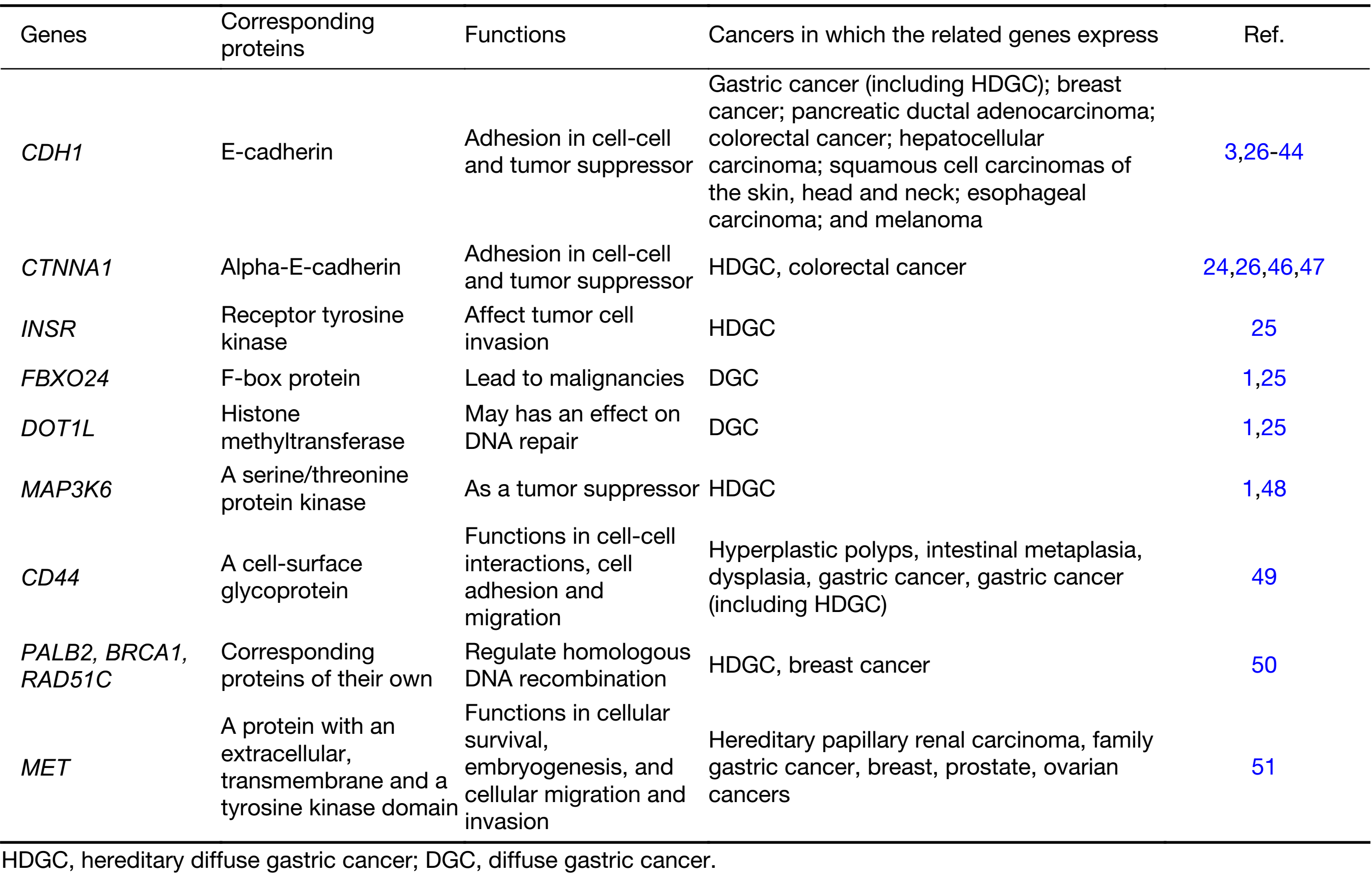
Full table
Strategies to gastric cancer patients with germline mutations
Patients with CDH1 mutation may get benefits from endoscopic surveillance to determine the time for surgery (52). NCCN guidelines recommends CDH1 pathogenic mutation carriers undergo prophylactic gastrectomy, however, individuals who reject such treatment may consider gastroscopic surveillance with multiple biopsies in every 6–12 months of interval after receiving genetic counseling. Additionally, Moreira et al. suggested that it is better to distinguish the starting time of surveillance between CDH1 mutation carriers (at the age of 20 years) and non-carriers (at the age of 40 years) (53). Regarding to the age of receiving prophylactic gastrectomy, NCCN recommends not earlier than 18 years in general, whereas IGCLC suggests the earliest optimal age as 20 years (54). Moreover, Moreira et al. and Tan et al. expressed the same opinions to IGCLC (53,55). Therefore, albeit different age limit was recommended by NCCN and IGCLC, the international consensus on the earliest age of individuals undertaking prophylactic gastrectomy is not younger than 18 years old. However, van der Post et al. advised that one should consider personal status and follow individualized manner to decide when to receive prophylactic gastrectomy (11). Moreover, the appropriate age for genetic testing for patients diagnosed as HDGC is at 18 years of age or earlier (4). Such strategy is also applicable to adolescent asymptomatic CDH1 mutation carriers. In the report written by Wickremeratne et al. (54), a total gastrectomy was performed on a 16-year-old asymptomatic CDH1 gene mutation carrier, and there were two normal results of gastroscopies with biopsies before the gastrectomy. Family history showed that the patient’s mother and aunt died on the age of 39 and 21 respectively, both because of gastric cancer. This case documented the youngest CDH1 carrier to date, who had a prophylactic gastrectomy, and who was several years younger than the age that guidelines recommended for the consideration of gastrectomy. However, multiple foci of early-stage carcinoma were found in her gastrectomy specimen. In another case, a 43-year-old female accepted genetic counseling and prophylactic total gastrectomy after the death of her brother and nephew (56). Although no evidence of dysplasia or early foci of SRCC was seen in 30 biopsies and 68 postoperative blocks, “prevention is better than cure” was the common consensus and final decision of both patients and families. Study has shown that bigger tumor size and younger age were associated with higher risk of recurrence of gastric cancer (57), therefore, receiving prophylactic gastrectomy at early age is likely to benefit patients with hereditary gastric cancer history and reduce recurrent risk, contributing to longer progression free survival time. In addition, Christgen et al. reported that CDH1 is related to ILC for the loss of E-cadherin, which is evidenced by a conditional knockout mouse model (15). Furthermore, the IGCLC suggests that albeit no DGC family history, patients with sporadic early onset LBCs are strongly recommended to germline screening for CDH1 especially the one with bilateral sign (9).
Although prophylactic surgery and regular surveillance might have some positive effects on decreasing the mortality rate of HDGCs, the psychological trauma and burden to the germline mutation carriers and their families could not be ignored. Therefore, genetic counseling plays a key role in providing patients and their families with humanistic care in order to facilitate appropriate management to different population. For the individuals who are diagnosed as malignant diseases at young age and/or without cancer family history, genetic counseling may potentially help them decide “the best” risk management strategies, choosing from prophylactic gastrectomy and routine endoscopic surveillances (58). Choi et al. reported that an individual with normal physical examination and positive family history obtained benefits from genetic counseling and chose the appropriate strategies for himself and his family members (44). However, decision-making period and process can be different from individuals who are pathogenic mutation carriers or at high risks, owing to a number of interrelated factors, such as objective risk confirmation, perceived familial cancer burden, subjective risk perceptions, experiences and perceptions of the different risk management options and life stage, etc. Therefore, the role of genetic counselors is more of importance to guide individuals to understand their risk status followed by making optimal risk management decisions (59). Additionally, guidelines for genetic counseling are also essential to be established to assist medical geneticists, genetic counselors, and other health-care providers in making decisions about appropriate management of genetic concerns (5). Moreover, the establishment of multi-disciplinary team (MDT) consisting of medical geneticists, genetic counselors, clinicians, pathologists, psychologists, etc. is also helpful to assess patients’ status in a comprehensive and professional manner, and may support them to make “the most appropriate” risk management decisions.
Molecular genetic testing and perspectives of future research regarding HDGC
Since 50%–70% of the families with HDGC have been reported as no identifiable CDH1 germline pathogenic variant, it is likely that some of these families may have pathogenic variants in other unidentified HDGC-susceptibility genes. To test CDH1 and other germline mutations, the following techniques are included: 1) sequence analysis: sequencing detects small intragenic deletions/insertions and missense, nonsense, as well as splice site variants. In general, polymerase chain reaction (PCR) based Sanger sequencing and direct sequencing were commonly applied; and 2) deletion/duplication analysis: exonic or whole-gene deletions/duplications are able to be detected by quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and chromosomal microarray analysis (CMA) which includes this gene/chromosome segment, such as Sanger sequencing and MLPA (60). PCR-direct sequencing and MLPA was used to evaluate the patients with negative sequencing results (61). Molinaro et al. investigated on CDH1 germline defects in 32 HDGC Italian probands who were selected according to international consensus criteria along with 5 randomly chosen relatives. They used a series of molecular methods to perform genetic testing, including: DNA sequencing, MLPA, single-nucleotide primer extension, bisulfite sequencing, reverse transcription-polymerase chain reaction (RT-PCR), and bioinformatics tools. Their data supported the need of a multi-method approach for CDH1 genetic testing, demonstrating that both DNA and RNA analyses are required to increase the detection rate of pathogenic mutations, thus reducing the number of patients without a clear molecular diagnosis (34). Another study reported by Majewski et al., using exome sequencing, mass spectrometry genotyping and candidate gene resequencing, demonstrated that CTNNA1 was detected as a HDGC susceptibility gene (24), indicating another classic method for seeking new candidate genes. Recently, a new approach utilizing bio-imaging analysis of in situ fluorescence microscopy has gradually arisen to quantify mutant E-cadherin (62). The expression level of E-cadherin was quantified and the distribution of the protein was characterized by a bio-imaging pipeline from in situ immunofluorescence images. By virtue of this new approach, the distinction of expressing mutant forms of E-cadherin displaying fluorescence profiles between mutant-type cells and wild-type cells was verified. The study illustrated that this method could be applied in evaluating the pathogenicity of E-cadherin missense variants as a complementary approach. Furthermore, this method could be accepted in detecting a wide range of proteins and some diseases featured by aberrant protein expression or trafficking.
Conclusions
To date, pathogenic CDH1 germline mutation has been found to be one of the major causes to HDGC, however, individuals carrying such mutation only take one fourth of the total HDGC population. Due to the hereditary susceptible trait, HDGC draws increasing attentions to the identification of pathogenic genes, especially on hotspots, mutation rate and penetrance of relevant germline mutations. With the help of rapid development of cutting edge genetic testing technologies and data analysis tools, genetic testing becomes more efficient and less costly, enabling more researchers to discover candidate susceptibility genes for hereditary cancer syndromes, however, more efforts should be put on family history collection and seeking valuable familial and hereditary cancer syndrome pedigrees. This precious information may potentially play key roles in uncovering new susceptibility genes, improving risk management and providing more choices for individuals carrying pathogenic mutations. Identification of new predisposing genes would give novel insights in the molecular pathogenesis of gastric cancer. Furthermore, one should consider difference between ethnic groups and geographical diversity when draft guidelines and standards. New precision medicine techniques like exome sequencing would also provide better tools for predisposing gene screening and early intervention possibilities for the mutation carriers, in addition to development of more reliable surveillance approaches to prevent unnecessary prophylactic gastric resection and to inform joint decision making. The concept of precision medicine was raised to promote individualized therapeutic strategies, and the core ideology of genetic counseling is to make personalized risk management plans which are tailored to specific individual. Genetic counseling for hereditary cancer syndrome including HDGC has met unprecedent opportunity. Studies have shown that decision-making for receiving prophylactic gastrectomy or routine surveillance was influenced by many aspects according to genetic counseling interview data. Thus, more studies on key factors that could have effect on risk management decision-making are required for the improvement of international guidelines and standards. Moreover, experienced and professional genetic counselors as well as a systematic MDT are also required to facilitate the development of genetic counseling and benefit pathogenic mutation carriers who are in need of regular and standardized risk management solutions.
Acknowledgements
This work was supported by Beijing Municipal Administration of Hospitals’ Youth Program (QML20151003), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201701) and Inner Mongolia Science & Technology Plan (kjt13sf04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol 2016;43:554–9. [PubMed] DOI:10.1053/j.seminoncol.2016.08.006
- Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery 2001;130:612–7; discussion 619–9. [PubMed] DOI:10.1067/msy.2001.117099
- Oliveira C, Bordin MC, Grehan N, et al. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat 2002;19:510–7. [PubMed] DOI:10.1002/humu.10068
- Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010;47:436–44. [PubMed] DOI:10.1136/jmg.2009.074237
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 2015;17:70–87. [PubMed] DOI:10.1038/gim.2014.147
- Benusiglio PR, Colas C, Rouleau E, et al. Hereditary diffuse gastric cancer syndrome: improved performances of the 2015 testing criteria for the identification of probands with a CDH1 germline mutation. J Med Genet 2015;52:563–5. [PubMed] DOI:10.1136/jmedgenet-2015-103153
- Shah MA, Salo-Mullen E, Stadler Z, et al. De novo CDH1 mutation in a family presenting with early-onset diffuse gastric cancer. Clin Genet 2012;82:283–7. [PubMed] DOI:10.1111/j.1399-0004.2011.01744.x
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1–10. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.01.01
- Corso G, Intra M, Trentin C, et al. CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 2016;15:215–9. [PubMed] DOI:10.1007/s10689-016-9869-5
- Dossus L, Benusiglio PR. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res 2015;17:37. [PubMed] DOI:10.1186/s13058-015-0546-7
- van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 2015;52:361–74. [PubMed] DOI:10.1136/jmedgenet-2015-103094
- Monahan KJ, Hopkins L. Diagnosis and management of hereditary gastric cancer. Recent Results Cancer Res 2016;205:45–60. [PubMed] DOI:10.1007/978-3-319-29998-3_4
- Sugimoto S, Komatsu H, Morohoshi Y, et al. Recognition of and recent issues in hereditary diffuse gastric cancer. J Gastroenterol 2015;50:831–43. [PubMed] DOI:10.1007/s00535-015-1093-9
- Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol 2015;1:23–32. [PubMed] DOI:10.1001/jamaoncol.2014.168
- Christgen M, Derksen P. Lobular breast cancer: molecular basis, mouse and cellular models. Breast Cancer Res 2015;17:16. [PubMed] DOI:10.1186/s13058-015-0517-z
- Kim HC, Wheeler JM, Kim JC, et al. The E-cadherin gene (CDH1) variants T340A and L599V in gastric and colorectal cancer patients in Korea. Gut 2000;47:262–7. [PubMed] DOI:10.1136/gut.47.2.262
- Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348–53. [PubMed] DOI:10.1053/gast.2001.29611
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007;297:2360–72. [PubMed] DOI:10.1001/jama.297.21.2360
- Benusiglio PR, Malka D, Rouleau E, et al. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: a multicentre study. J Med Genet 2013;50:486–9. [PubMed] DOI:10.1136/jmedgenet-2012-101472
- Oliveira C, Senz J, Kaurah P, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet 2009;18:1545–55. [PubMed] DOI:10.1093/hmg/ddp046
- Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med 2016;31:1042–53. [PubMed] DOI:10.3904/kjim.2016.147
- Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392:402–5. [PubMed] DOI:10.1038/32918
- Jonsson BA, Bergh A, Stattin P, et al. Germline mutations in E-cadherin do not explain association of hereditary prostate cancer, gastric cancer and breast cancer. Int J Cancer 2002;98:838–43. [PubMed] DOI:10.1002/ijc.10258
- Majewski IJ, Kluijt I, Cats A, et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol 2013;229:621–9. [PubMed] DOI:10.1002/path.4152
- Donner I, Kiviluoto T, Ristimäki A, et al. Exome sequencing reveals three novel candidate predisposition genes for diffuse gastric cancer. Fam Cancer 2015;14:241–6. [PubMed] DOI:10.1007/s10689-015-9778-z
- Gall TM, Frampton AE. Gene of the month: E-cadherin (CDH1). J Clin Pathol 2013;66:928–32. [PubMed] DOI:10.1136/jclinpath-2013-201768
- Keller G. Hereditary aspects of gastric cancer. Pathologica 2002;94:229–33. [PubMed] DOI:10.1007/s102420200037
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol 2015;17:533–9. [PubMed] DOI:10.1038/ncb3136
- Bianchini JM, Kitt KN, Gloerich M, et al. Reevaluating αE-catenin monomer and homodimer functions by characterizing E-cadherin/αE-catenin chimeras. J Cell Biol 2015;210:1065–74. [PubMed] DOI:10.1083/jcb.201411080
- Huels DJ, Ridgway RA, Radulescu S, et al. E-cadherin can limit the transforming properties of activating β-catenin mutations. EMBO J 2015;34:2321–33. [PubMed] DOI:10.15252/embj.201591739
- Suriano G, Yew S, Ferreira P, et al. Characterization of a recurrent germ line mutation of the E-cadherin gene: implications for genetic testing and clinical management. Clin Cancer Res 2005;11:5401–9. [PubMed] DOI:10.1158/1078-0432.CCR-05-0247
- More H, Humar B, Weber W, et al. Identification of seven novel germline mutations in the human E-cadherin (CDH1) gene. Hum Mutat 2007;28:203. [PubMed] DOI:10.1002/humu.9473
- Mayrbaeurl B, Keller G, Schauer W, et al. Germline mutation of the E-cadherin gene in three sibling cases with advanced gastric cancer: clinical consequences for the other family members. Eur J Gastroenterol Hepatol 2010;22:306–10. [PubMed] DOI:10.1097/MEG.0b013e32832bab9a
- Molinaro V, Pensotti V, Marabelli M, et al. Complementary molecular approaches reveal heterogeneous CDH1 germline defects in Italian patients with hereditary diffuse gastric cancer (HDGC) syndrome. Genes Chromosomes Cancer 2014;53:432–45. [PubMed] DOI:10.1002/gcc.22155
- Sugimoto S, Yamada H, Takahashi M, et al. Early-onset diffuse gastric cancer associated with a de novo large genomic deletion of CDH1 gene. Gastric Cancer 2014;17:745–9. [PubMed] DOI:10.1007/s10120-013-0278-2
- López M, Cervera-Acedo C, Santibáñez P, et al. A novel mutation in the CDH1 gene in a Spanish family with hereditary diffuse gastric cancer. Springerplus 2016;5:1181. [PubMed] DOI:10.1186/s40064-016-2852-7
- Humar B, Toro T, Graziano F, et al. Novel germline CDH1 mutations in hereditary diffuse gastric cancer families. Hum Mutat 2002;19:518–25. [PubMed] DOI:10.1002/humu.10067
- Brooks-Wilson AR, Kaurah P, Suriano G, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 2004;41:508–17. [PubMed] DOI:10.1136/jmg.2004.018275
- Norton JA, Ham CM, Van Dam J, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg 2007;245:873–9. [PubMed] DOI:10.1097/01.sla.0000254370.29893.e4
- Frebourg T, Oliveira C, Hochain P, et al. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet 2006;43:138–42. [PubMed] DOI:10.1136/jmg.2005.031385
- Suriano G, Oliveira C, Ferreira P, et al. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum Mol Genet 2003;12:575–82. [PubMed] DOI:10.1093/hmg/ddg048
- Han MA, Oh MG, Choi IJ, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol 2012;30:701–8. [PubMed] DOI:10.1200/jco.2011.35.3078
- Figueiredo J, Söderberg O, Simões-Correia J, et al. The importance of E-cadherin binding partners to evaluate the pathogenicity of E-cadherin missense mutations associated to HDGC. Eur J Hum Genet 2013;21:301–9. [PubMed] DOI:10.1038/ejhg.2012.159
- Choi HJ, Ki CS, Suh SP, et al. Presymptomatic identification of CDH1 germline mutation in a healthy Korean individual with family history of gastric cancer. Ann Lab Med 2014;34:386–9. [PubMed] DOI:10.3343/alm.2014.34.5.386
- Sun GY, Wu JX, Wu JS, et al. Caveolin-1, E-cadherin and β-catenin in gastric carcinoma, precancerous tissues and chronic non-atrophic gastritis. Chin J Cancer Res 2012;24:23–8. [PubMed] DOI:10.1007/s11670-012-0023-0
- Lynch HT, Grady W, Suriano G, et al. Gastric cancer: new genetic developments. J Surg Oncol 2005;90:114–33; discussion 133. [PubMed] DOI:10.1002/jso.20214
- Sygut A, Przybylowska K, Ferenc T, et al. Genetic variations of the CTNNA1 and the CTNNB1 genes in sporadic colorectal cancer in Polish population. Pol Przegl Chir 2012;84:560–4. [PubMed] DOI:10.2478/v10035-012-0093-1
- Gaston D, Hansford S, Oliveira C, et al. Germline mutations in MAP3K6 are associated with familial gastric cancer. PLoS Genet 2014;10:e1004669. [PubMed] DOI:10.1371/journal.pgen.1004669
- da Cunha CB, Oliveira C, Wen X, et al. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest 2010;90:1604–14. [PubMed] DOI:10.1038/labinvest.2010.155
- Sahasrabudhe R, Lott P, Bohorquez M, et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology 2017;152:983–6.e6. [PubMed] DOI:10.1053/j.gastro.2016.12.010
- Kim IJ, Park JH, Kang HC, et al. A novel germline mutation in the MET extracellular domain in a Korean patient with the diffuse type of familial gastric cancer. J Med Genet 2003;40:e97. [PubMed] DOI:10.1136/jmg.40.8.e97
- Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc 2017. pii: S0016-5107(17)32076-X.
- Moreira L, Castells A. Surveillance of patients with hereditary gastrointestinal cancer syndromes. Best Pract Res Clin Gastroenterol 2016;30:923–35. [PubMed] DOI:10.1016/j.bpg.2016.10.004
- Wickremeratne T, Lee CH, Kirk J, et al. Prophylactic gastrectomy in a 16-year-old. Eur J Gastroenterol Hepatol 2014;26:353–6. [PubMed] DOI:10.1097/MEG.0000000000000016
- Tan RY, Ngeow J. Hereditary diffuse gastric cancer: What the clinician should know. World J Gastrointest Oncol 2015;7:153–60. [PubMed] DOI:10.4251/wjgo.v7.i9.153
- Daunton A, Puig S, Taniere P, et al. Prevention is better than cure. J Surg Case Rep 2012;2012:14. [PubMed] DOI:10.1093/jscr/2012.6.14
- Shin CH, Lee WY, Hong SW, et al. Characteristics of gastric cancer recurrence five or more years after curative gastrectomy. Chin J Cancer Res 2016;28:503–10. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.05.05
- Feroce I, Serrano D, Biffi R, et al. Hereditary diffuse gastric cancer in two families: A case report. Oncol Lett 2017;14:1671–4. [PubMed] DOI:10.3892/ol.2017.6354
- Hallowell N, Badger S, Richardson S, et al. An investigation of the factors effecting high-risk individual’ decision-making about prophylactic total gastrectomy and surveillance for hereditary diffuse gastric cancer (HDGC). Fam Cancer 2016;15:665–76. [PubMed] DOI:10.1007/s10689-016-9910-8
- Petridis C, Shinomiya I, Kohut K, et al. Germline CDH1 mutations in bilateral lobular carcinoma in situ. Br J Cancer 2014;110:1053–7. [PubMed] DOI:10.1038/bjc.2013.792
- Kim S, Chung JW, Jeong TD, et al. Searching for E-cadherin gene mutations in early onset diffuse gastric cancer and hereditary diffuse gastric cancer in Korean patients. Fam Cancer 2013;12:503–7. [PubMed] DOI:10.1007/s10689-012-9595-6
- Sanches JM, Figueiredo J, Fonseca M, et al. Quantification of mutant E-cadherin using bioimaging analysis of in situ fluorescence microscopy. A new approach to CDH1 missense variants. Eur J Hum Genet 2015;23:1072–9. [PubMed] DOI:10.1038/ejhg.2014.240
