Treatment patterns for adjuvant docetaxel-based chemotherapy in early-stage breast cancer in China: A pooled retrospective analysis of four observational studies
Introduction
Breast cancer is the most common cancer among women globally and in China, and represents a leading cause of mortality and morbidity (1,2). However, early-stage breast cancer (EBC) is potentially curable with surgery and appropriate adjuvant systemic therapy to reduce the risk of distant recurrance (3). Epidemiological data suggest that the majority of breast cancer cases in China are clinical stage I and II, and surgery is the most common treatment; received by approximately 99.6% of women with breast cancer (4). Chinese women with breast cancer have different characteristics to their Western counterparts, including a relatively young age at diagnosis, different tumor pathologies and different treatment safety profiles, all of which influence treatment selection (4,5).
Adjuvant systemic therapy is recommended by most breast cancer treatment guidelines (6-9), largely based on evidence from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) study, which found a reduced risk of recurrence and death for adjuvant chemotherapy and endocrine therapy (10,11). The decision to use adjuvant therapy is based on several factors including the predicted benefit of adjuvant use and the risk of recurrence as assessed by several parameters including patients’ age and health, tumor stage, tumor subtype and genetic profile [estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2), and Ki67 expression status]. Although several scoring tools are available to aid treatment decision making, the final assessment of patient suitability for adjuvant chemotherapy is made by oncologists on an individual basis (3).
Epidemiological data from China show that since 2006 adjuvant chemotherapy regimens containing both anthracyclines (doxorubicin or epirubicin) and taxanes (docetaxel or paclitaxel) have become the most commonly used, with epirubicin and docetaxel being the respective treatments of choice (4). Anthracyclines have demonstrated signficant reductions in the risk of recurrence and overall mortality in EBC compared with non-anthracycline regimens such as cyclophosphamide plus methotrexate and 5-fluoruracil (10,12,13). However, these agents are associated with cardiotoxicities including irreversible congestive heart failure, and their use in the West has declined in recent years in favor of taxane-based treatments (13,14).
Taxane-based adjuvant chemotherapy with docetaxel or paclitaxel reduces the risk of recurrence and death in EBC compared with taxane-free chemotherapy (15,16). Compared with paclitaxel, docetaxel has higher pharmacokinetic activity in vitro and can be administered concurrently with doxorubicin without associated cardiotoxicity (3,17). Furthermore, recent evidence suggests docetaxel is associated with superior disease-free survival (DFS) versus paclitaxel in the adjuvant setting for EBC (18). The taxane toxicity profile varies according to the dosing schedule, with more neutropenia reported with three-weekly docetaxel; however, the majority of adverse effects (AEs) can be managed through treatment and supportive care (19-21).
Treatment guidelines provide detailed algorithms for selecting adjuvant treatments for EBC (6,9). The selection of individual docetaxel-based regimens is made based on tumor-specific factors including tumor size and biology, and also patient-specific factors including age, health and risk of recurrence (3,6,7,9). A variety of docetaxel-containing adjuvant treatment regimens utilizing different combinations of therapeutics and dosing strategies have been investigated, all of which have different risk/benefit profiles. Docetaxel plus cyclophosphamide (TC) and docetaxel plus doxorubicin and cyclophosphamide (TAC) were shown to have superior survival versus non-docetaxel-containing regimens for adjuvant treatment of EBC as well as metastatic breast cancer (22-26). In women with node-positive EBC, a sequential docetaxel regimen [doxorubicin and cyclophosphamide followed by docetaxel (AC-T)] was associated with superior survival outomes compared with concurrent doxorubicin and docetaxel (TA) and concurrent TAC (27). However, data from a 10-year follow-up of a further study revealed no long-term difference in DFS or overall survival (OS) with sequential versus concurrent docetaxel (28).
Given that most evidence for docetaxel-based regimens has been reported from controlled clinical trials, and considering the wide range of adjuvant treatment strategies available, it is important to understand the use of adjuvant docetaxel in real-world clinical practice. The Asia-Pacific Breast Initiatives (APBI) I and II were set up to collect, analyze and disseminate data on the real-world use of adjuvant docetaxel for EBC in the Asia-Pacific region (29-31). The analysis presented in this report pooled data from Chinese patients included in APBI I and II as well as two Chinese breast cancer registries to investigate patterns of adjuvant docetaxel use for EBC in real-life clinical practice in China.
Materials and methods
Study design and patients
This was a retrospective, pooled analysis of the APBI I (2006–2008) and II (2009–2011) registries (29,30), and two Chinese observational studies; BC Local Registry (2007–2010) and BC STATE (2011–2014). The present analysis included female Chinese adults (≥18 years) enrolled into these four observational studies with operable breast cancer who were treated with docetaxel-based adjuvant chemotherapy. Patients with metastatic disease were excluded.
APBI I and II were international phase IV, observational, open-label, multicenter studies that prospectively enrolled patients with operable early breast cancer who had a high- (APBI I) or intermediate-high (APBI II) risk of recurrence and received adjuvant docetaxel. Exclusion criteria were billirubin >upper limit of normal (ULN), serum glutamic oxaloacetic transaminase (SGOT) and/or serum glutamic pyruvic transaminase (SGPT) >1.5×ULN concomitant with alkaline phosphatase >2.5×ULN, neutrophil counts of <1,500 cells/mm3 and history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Safety data were collected after 1 year of follow-up for the APBI I registry and after 1.5 years follow-up for the APBI II registry.
The BC Local Registry was a retrospective, multicenter observational study which aimed to assess patient profiles, patterns of care, and treatment outcome in patients with operable breast cancer treated with docetaxel-based adjuvant chemotherapy. Patients were excluded if they had bilirubin >ULN, SGOT >1.5×ULN with alkaline phosphatase (ALP) >2.5×ULN, or glutamic-pyruvic transaminase (GPT) >1.5×ULN with ALP >2.5×ULN. Patients with ongoing docetaxel treatment were also excluded. BC STATE was an observational study that aimed to assess the patterns of treatment in patients with operable newly-diagnosed breast cancer who received docetaxel-based adjuvant chemotherapy at Tier I non-cancer-specialized hospitals and other city hospitals in China.
For all patients, treatment was selected at the discretion of the treating physician. Written informed consent was obtained from patients before entry to all studies. The pooled data were grouped by treatment selection with four groups defined as: patients receiving docetaxel and anthracycline in combination (docetaxel/anthracycline combo) or sequentially (docetaxel/anthracycline sequential); docetaxel in combination with other medication (docetaxel/other); or “other” treatment. Patients who switched treatment once or more during the chemotherapy period were grouped according to the first treatment received.
Study endpoints and data collection
The primary analysis endpoint was to assess treatment patterns and patient profiles for docetaxel-based adjuvant chemotherapy for EBC in real-world clinical practice in China. Secondary endpoints included assessment of the tolerability and safety of docetaxel-based adjuvant chemotherapy as well as investigating baseline factors (age, height, weight, menopausal status, concomitant disease, tumor phase, tumor type, and estrogen, progesterone and biomarker-HER2 status) associated with treatment selection.
Tumor molecular subtypes were defined according to the current guidelines at the time of patient enrolment. Since 2011, tumors with 1% nuclear-stained cells have been classified as ER and/or progesterone receptor (PR) positive according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines (32). Prior to this, tumors with 10% nuclear-stained cells were defined as ER and/or PR positive. HER2 staining was evaluated from 0 to 3+ according to the ASCO/CAP guidelines; 3+ was considered positive while 0 and 1+ were considered negative (33). Samples with HER2 2+ were confirmed by fluorescent in situ hybridisation (FISH).
AEs were defined as treatment-emergent (TEAE) if the AE started on or after the date of the first dose of study drug up to and including 21 d after the last dose of study drug, or if the AE started before the first dose of study drug but worsened in severity on or after the date of first dose of study drug, up to and including 21 d after last dose of study drug. Subjects experiencing the same AE more than once over the course of the study were counted only once in the incidence calculation for that AE.
Statistical analysis
Continuous variables were summarized as x±s unless specified. Categorical variables were summarized using frequency and percentage. The Chi-square test was used for multiple proportion comparisons. Univariate and multivariate logistic regression models were fitted to examine baseline factors associated with treatment selection. The level of significance was set at P<0.05 and all analyses were performed using SAS software (Version 9.2 or above; SAS Institute Inc., Cary, NC, USA).
Results
Patients
In total, data from 3,020 patients were included; from the BC Local Registry [1,913 (63.3%)], BC STATE [496 (16.4%)], APBC I [(329 (10.9%)], and APBC II [282 (9.3%)] studies. The majority of study centers were located in Guangdong [19 (16.2%)], Beijing [18 (15.4%)], Zhejiang [15 (12.8%)], Shanghai [14 (12.0%)] and Jiangsu [12 (10.3%)] (Supplementary Table S1). The highest number of patients were enrolled from Beijing [729 (24.14%)], Shanghai [488 (16.16%)], Zhejiang [456 (15.10%)] and Jiangsu [399 (13.21%)] (Supplementary Table S2). Of the patients included in this pooled analysis, 1,421 (47.1%) received docetaxel/anthracycline combo, 447 (14.8%) received docetaxel/anthracycline sequential, 705 (23.3%) received docetaxel/other, and 447 (14.8%) were treated with “other” chemotherapy regimens. Overall, 1,868 (61.9%) subjects received an anthracycline-based regimen and 1,152 (38.1%) subjects received a non-anthracycline-based regimen.

Full table

Full table
The mean age of all study subjects was 49.3±9.5 years and was similar across the treatment categories (Table 1). Overall, 14.9% of subjects were in the age group of <40 years, 78.4% were in the age group of 40–65 years, and 6.0% were aged ≥65 years. The majority of patients were pre-menopausal, with the highest proportion of pre-menopausal patients in the docetaxel/anthracycline combo group (60.1%).

Full table
Total mastectomy was the most common type of surgical intervention across all groups. Patients in the docetaxel/anthracycline sequential group had a higher proportion of partial mastectomy (20.8%), breast reconstruction (50.3%) and lymph node excision (91.9%) compared with all other treatment groups. Neoadjuvant chemotherapy was received by 13.3% of patients overall, with the highest use observed for patients in the docetaxel/anthracycline combo group (17.7%), and the lowest use among patients in the docetaxel/anthracycline sequential group (0%).
Tumor classification and pathology
The most common tumor stage in all treatment groups was stage IIA (32.4%), and the majority of all patients were stage IIA–IIIA (75.8%) (Table 2). Among all patients, over 80% had ductal carcinoma, 68.6% had tumor size ≥2 cm, 12.6% had lymphovascular invasion and the most common molecular subtypes were Luminal A (35.3%) and triple-negative (21.2%).

Full table
Almost all patients had ER, PR and HER2 testing results (Table 2). The majority (61.9%) of patients were ER positive, 36.0% were ER negative and 2.0% subjects had unknown status. For PR, the majority (54.8%) of patients were positive, 42.9% were negative and 2.2% had unknown status. Only a small number (476) of patients had HER2/neu status evaluated by FISH. However, of the majority who had HER2/neu status evaluated by immunohistochemistry, the most common HER2/neu status was 0 (32.9%), followed by 2+ (17.8%), 1+ (17.3%) and 3+ or greater (15.6%).
Use of docetaxel-based adjuvant therapy
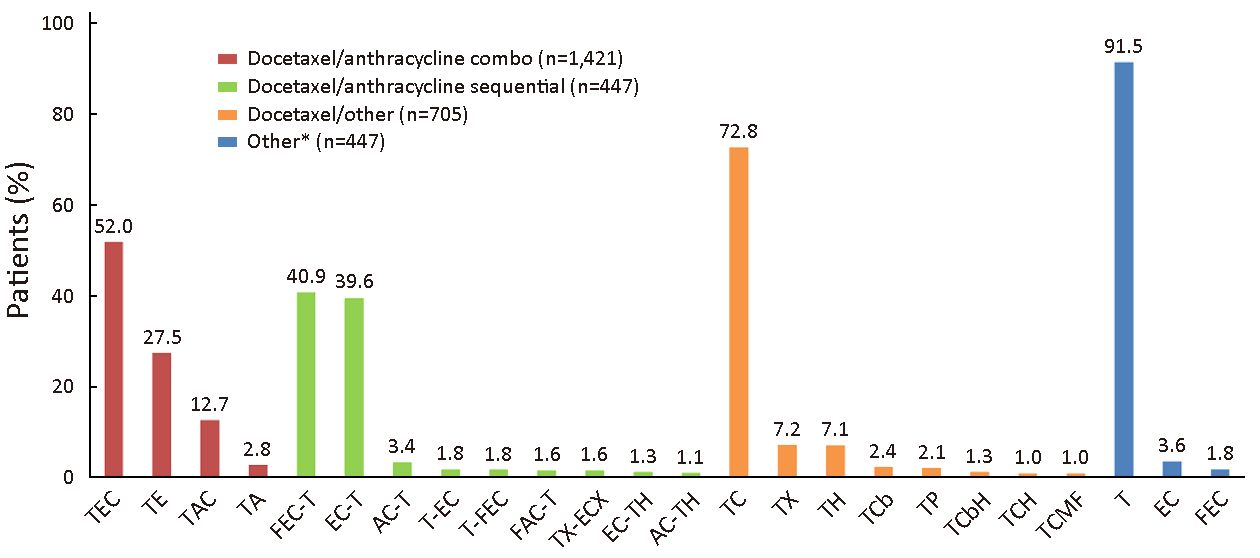
Among all patients included in the study, the most commonly used adjuvant chemotherapy regimens were T/epirubicin (E)/C (TEC) [739 (24.5%)], TE [391 (12.9%)] and TAC [180 (6.0%)]. Of the confirmed docetaxel/anthracycline combo regimens the most commonly used was TEC (52.0%), followed by TE (27.5%) and TAC (12.7%) (Figure 1). Among patients in the docetaxel/anthracycline sequential group, 40.9% and 39.6% received 5-flurouracil (F)/EC-T and EC-T, respectively. For patients in the docetaxel/other group, 72.8% received TC, and for those in the “other” group 91.5% received docetaxel monotherapy.
There was a wide variation in the number of docetaxel cycles and doses received between the treatment groups (Table 3). Patients in the docetaxel/anthracycline combo group received the highest number of cycles of docetaxel (5.3) and the highest total docetaxel dose (385.3 mg/m2) when compared with all other groups. In contrast, patients in the docetaxel/anthracycline sequential group received the lowest number of cycles (3.4) and total dose (277.5 mg/m2) of docetaxel, although the average docetaxel dose per cycle for these patients was the highest of all treatment groups (80.9 mg/m2 per cycle).
Baseline factors associated with treatment selection
Multivariate regression analysis revealed that patients were more likely to receive docetaxel/anthracycline combo therapy if they were: 1) aged <40 years versus ≥65 years (P<0.001); 2) had lower bodyweight (P=0.049); 3) were pre-menopausal versus post-menopausal (P<0.001); 4) had an unknown ER status versus being ER negative (P=0.005); or 5) if they had lobular versus ductal carcinoma (P<0.001) (Table 4). A docetaxel/antracycline sequential regimen was more likely to be selected for patients aged <40 years versus ≥65 years (P=0.008) and ER negative patients (P=0.002) (Table 5). Furthermore, significant associations were found between patients receiving docetaxel/other therapy and being aged ≥65 years versus <40 years (P<0.001), being post- versus pre-menopausal (P=0.007) and having positive versus negative PR status (P<0.001) (Table 6). HER2 status was excluded from the analyses due to an insufficient number of patients with available data.

Full table
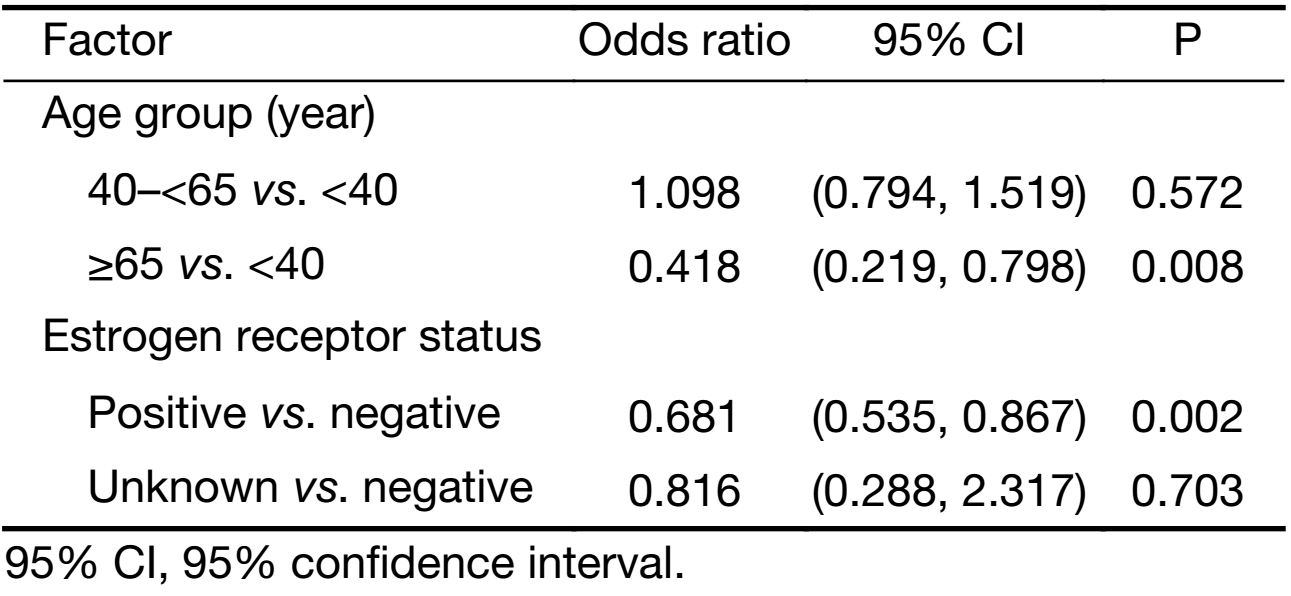
Full table
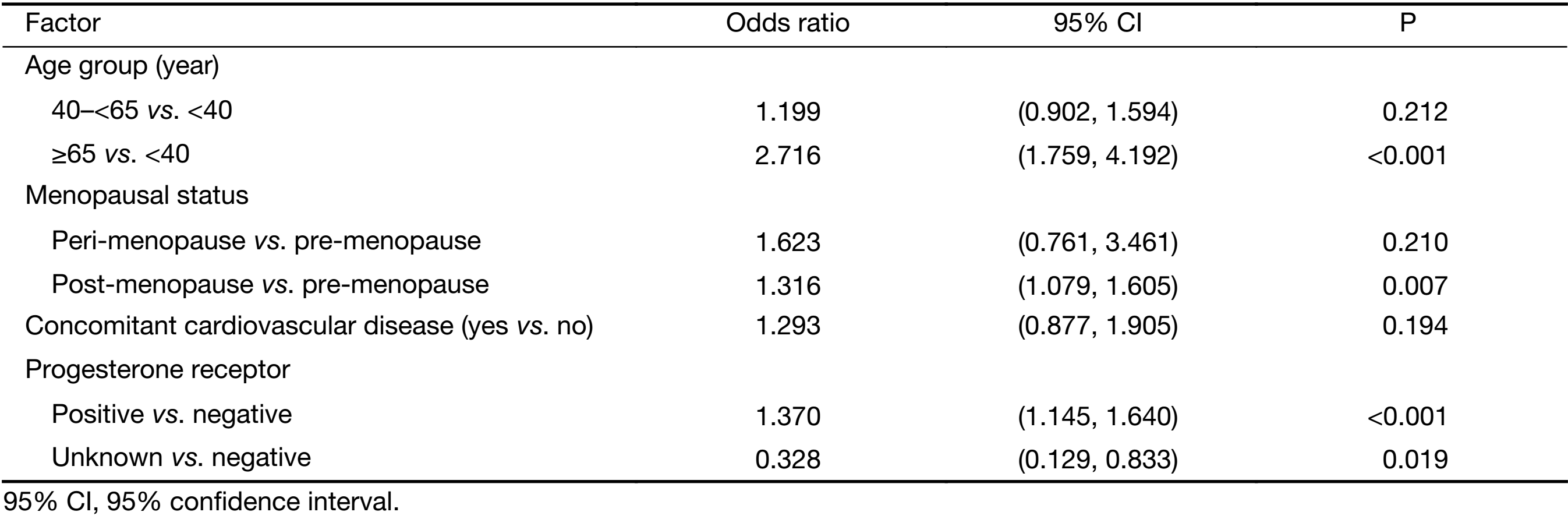
Full table
Safety
TEAEs were reported in 76.9%, 65.5%, 74.5% and 62.0% of patients in the docetaxel/anthracycline combo, docetaxel/anthracycline sequential, docetaxel/other and “other” treatment groups, respectively (Table 7). The majority of TEAEs were grade 1 and 2, with no grade 5 TEAS reported. Grade 3 TEAEs were most frequently reported in the docetaxel/anthracycline combo group, while grade 4 TEAEs were most common in the docetaxel/anthracycline combo and docetaxel/other groups (11.1% and 11.6%) compared with the docetaxel/anthracycline sequential and “other” treatment groups (7.8% and 7.6%). In addition, serious TEAEs were more frequently reported in the docetaxel/anthracycline combo and “other” treatment groups (9.7% and 9.6%) compared with the other two treatment groups. Hematological TEAEs were most commonly reported for patients in the docetaxel/anthracycline combo group (65.9%) and were least common in the docetaxel/anthracycline sequential group (36.7%). The overall incidence of cardiovascular TEAEs was low, with the highest incidence reported in the docetaxel/anthracycline group (1.1%).

Full table
Adherence to treatment guidelines
The chemotherapy treatment received by most patients was consistent with their planned treatment (Table 8). There was a statistically significant difference in the number of patients who received treatment in-line with the planned treatment between Tier 1 city hospitals versus other city hospitals (all P<0.05;Table 1).
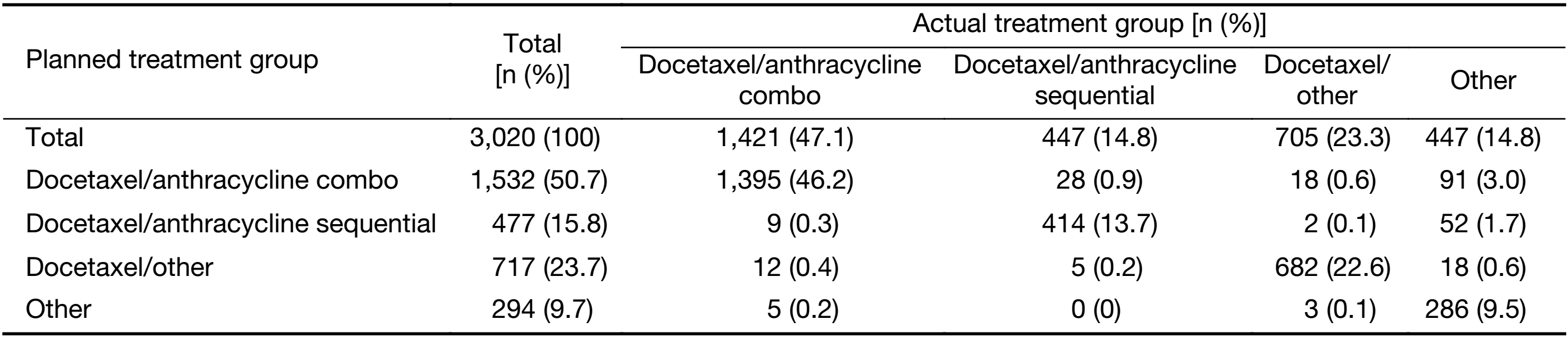
Full table
Discussion
Taxane-based adjuvant chemotherapy with docetaxel or paclitaxel for EBC has been extensively studied and is recommended by most international treatment guidelines (6,9-11,15,16,34). This pooled analysis of 3,020 patients from four observational studies addresses an important knowledge gap on treatment patterns and real-world use of docetaxel for EBC in China. The results indicate that the most commonly used treatment regimen category in this setting is docetaxel administered concurrently with anthracycline, which was received by almost half of the patient population (47.1%). This was followed by docetaxel combined with other agents, docetaxel and anthracycline administered sequentially, and “other” treatments primarily comprised of docetaxel monotherapy. The results also showed that overall the most commonly used docetaxel-based adjuvant chemotherapy regimens for EBC in China are TEC (24.5%), TE (12.9%) and TAC (6.0%).
The addition of taxanes to anthracycline-based chemotherapy has been shown to reduce breast cancer mortality, with improved DFS and OS (10,34). A meta-analysis of three phase III studies in EBC showed that sequential administration of anthracyclines and taxanes provides a significant improvement in in OS and DFS over combination dosing (35). In contrast, a long-term follow-up from the Breast Cancer International Research Group (BCIRG)-005 study suggested there is no difference in OS or DFS between TAC or AC-T, although TAC was associated with higher rates of febrile neutropenia, and AC-T with higher rates of myalgia, hand-foot syndrome, and neuropathy (28). Nonetheless, sequential administration of anthracyclines and taxanes is recommended by treatment guidelines (6,9). The results of the present study indicate that combination administration of docetaxel and anthracyclines is notably more common than sequential administration among EBC patients in China (47.1% vs. 14.8%). The choice of combination regimens may be based on several factors including improved patient compliance with the shorter overall treatment time, and the potential for synergistic treatment effects despite dose reductions required for combination use and an increased risk of toxicities (3). Although sequential treatment may have greater therapeutic benefit, this needs to be weighed against the risk of patient non-adherence. This explanation is supported by the relatively low average age of patients in this study compared with that commonly observed for Western EBC patient populations (49.3 years) and the low prevalence of concomitant cardiovascular disease (4.8%), which may have favored the use of a combination regimen.
The present analysis revealed that the majority of Chinese patients with EBC treated with docetaxel received anthracycline-based treatment (61.9%). This is interesting given that anthracycline-based regimens are usually reserved for patients at higher risk of disease recurrence, taking into consideration the potential cardiotoxic effects of these regimens (3,12). Accordingly, in the West the use of anthracyclines has been declining over the past two decades (13,14). Epidemiological data from China indicate the use of anthracycline-based adjuvant treatments without taxanes is declining (55% in 2003 vs. 25% in 2008), overtaken by taxane-based treatments with or without anthracyclines (4). In the Phase III US Oncology Research Trial 9735, four cycles of TC yielded superior DFS and OS compared to standard AC, and was tolerable in both older and younger EBC patients (22). However, a recent joint efficacy analysis of three large EBC trials showed that the addition of taxanes to AC significantly increased invasive-DFS compared to six cycles of TC alone (88.2% vs. 90.7%, P=0.04) (36). Nonetheless, the overall body of evidence suggests that anthracycline-free, taxane-based treatments are a feasible alternative in selected patients, particularly those at risk of cardiac complications and/or those with low- to moderate-risk disease (3,6,9,37).
The choice between sequential and combination therapy in this study was significantly associated with several patient baseline factors. Patients who are more likely to receive docetaxel/anthracycline combo treatment were those: 1) aged <40 years versus ≥65 years; 2) had lower bodyweight; 3) were pre- versus post-menopausal; 4) had an unknown ER status versus being ER negative, and 5) had lobular versus ductal carcinoma. Sequential treatment was more likely to be selected for patients aged <40 years versus ≥65 years, but selection was also associated with patients who were ER negative. These findings may reflect that older age is associated with better prognosis compared to younger patients, who have higher recurrence rates and a higher prevalence of factors associated with worse survival outcomes, including ER-negative disease (38,39). Adjuvant chemotherapy with an anthracycline-taxane regimen is usually recommended for patients with triple-negative, HER2-positive EBC and in those with high-risk HER2-negative tumors (3,6,8-10). However, in a recent EBCTCG meta-analysis, the relative benefit of anthracycline- or taxane-based chemotherapy was found to be similar in all patient subgroups independent of age, stage, histopathological grade and ER status, with treatment reducing mortality by one-third (10).
Although treatment guidelines recommend taxanes in combination with other agents for adjuvant treatment of EBC, in this study the vast majority of patients in the “other” treatment group received docetaxel monotherapy (91.5%) (6-9). Treatment with docetaxel alone is usually reserved for locally advanced or metastatic breast cancer in patients who have received prior chemotherapy (40,41). There are two likely explanations for the high proportion of patients in this study receiving docetaxel monotherapy. Firstly, the majority of patients (63.3%) were from the BC Local Registry which included retrospective data from patients treated many years ago when docetaxel monotherapy was relatively common in China. Secondly, if patients receiving EC-T were enrolled after completion of EC, they may have been recorded as receiving docetaxel monotherapy. However, with regards to general adherence to clinical guidelines, the actual chemotherapy treatment received by the majority of patients was consistent with their planned treatment, with only a limited number from each group switching to alternate chemotherapies. In addition, compliance with planned treatment was significantly higher at Tier 1 city hospitals compared with other city hospitals (all P<0.05).
This study had several limitations which deserve to mention. Firstly, this was a retrospective pooled analysis which has inherent limitations based on the individual studies and data sets used. In addition, the registry studies this analysis was based on had the inherent limitations of all observational studies including broad patient inclusion criteria, missing data, and a wide range of treatment regimens, which makes drawing strong conclusions difficult. Secondly, data on tumor staging characteristics including size, grading and lymphovascular invasion, as well as HER2 status by FISH testing, were not available for a large number of patients. These factors are known to influence treatment selection and their inclusion may have provided further insights into treatment patterns in this population. Similarly, an analysis of survival outcomes was not possible due to the limited number of patients with available data.
Conclusions
This pooled analysis provides valuable data from real-world clinical practice regarding adjuvant docetaxel treatment patterns in patients with operable breast cancer in China between 2006 and 2014. The most commonly-used docetaxel-based adjuvant therapy was the combined administration of docetaxel with anthracyclines, in particular the TEC regimen, reflecting the local preference for epirubicin over doxorubicin and combination administration over sequential treatment. The use of docetaxel/anthracycline combo therapy was associated with patient age, bodyweight, menopausal status, ER status, and tumor type (lobular versus ductal carcinoma). Taxane-based anthracycline-free regimens were the second most commonly used, which is consistent with the declining use of anthracyclines in both China and the West.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [PubMed] DOI:10.1016/s1470-2045(13)70567-9
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [PubMed] DOI:10.1002/ijc.29210
- Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med 2015;13:195. [PubMed] DOI:10.1186/s12916-015-0439-8
- Li Q, Yang Z, Fan J, et al. A nation-wide multicenter 10-year (1999-2008) retrospective study of chemotherapy in Chinese breast cancer patients. Oncotarget 2017;8:75864–73. [PubMed] DOI:10.18632/oncotarget.16439
- Chow LWC, Biganzoli L, Leo AD, et al. Toxicity profile differences of adjuvant docetaxel/cyclophosphamide (TC) between Asian and Caucasian breast cancer patients. Asia Pac J Clin Oncol 2017;13:372–8. [PubMed] DOI:10.1111/ajco.12682
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8–30. [PubMed] DOI:10.1093/annonc/mdv298
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw 2017;15:433–51. [PubMed] DOI:10.6004/jnccn.2017.0044
- Henry NL, Somerfield MR, Krop IE. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: American society of clinical oncology endorsement of cancer care Ontario guideline recommendations summary. J Clin Oncol 2016;34:2303–11. [PubMed] DOI:10.1200/JCO.2015.65.8609
- Denduluri N, Somerfield MR, Eisen A, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2) -negative and adjuvant targeted therapy for HER2-positive breast cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol 2016;34:2416–27. [PubMed] DOI:10.1200/JCO.2016.67.0182
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. [PubMed] DOI:10.1016/s0140-6736(11)61625-5
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–717. [PubMed] DOI:10.1016/S0140-6736(05)66544-0
- Crozier JA, Swaika A, Moreno-Aspitia A. Adjuvant chemotherapy in breast cancer: To use or not to use, the anthracyclines. World J Clin Oncol 2014;5:529–38. [PubMed] DOI:10.5306/wjco.v5.i3.529
- Giordano SH, Lin YL, Kuo YF, et al. Decline in the Use of Anthracyclines for Breast Cancer. J Clin Oncol 2012;30:2232–9. [PubMed] DOI:10.1200/JCO.2011.40.1273
- Greene J, Hennessy B. The role of anthracyclines in the treatment of early breast cancer. J Oncol Pharm Pract 2015;21:201–12. [PubMed] DOI:10.1177/1078155214531513
- Qin YY, Li H, Guo XJ, et al. Adjuvant chemotherapy, with or without taxanes, in early or operable breast cancer: a meta-analysis of 19 randomized trials with 30698 patients. PLoS One 2011;6:e26946. [PubMed] DOI:10.1371/journal.pone.0026946
- Ferguson T, Wilcken N, Vagg R, et al. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 2007;4:Cd004421. [PubMed] DOI:10.1002/14651858.CD004421.pub2
- Crown J, O’Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 2004;9 Suppl 2:24–32. [PubMed] DOI:10.1634/theoncologist.9-suppl_2-24
- Watanabe T, Kuranami M, Inoue K, et al. Comparison of an AC-taxane versus AC-free regimen and paclitaxel versus docetaxel in patients with lymph node-positive breast cancer: Final results of the National Surgical Adjuvant Study of Breast Cancer 02 trial, a randomized comparative phase 3 study. Cancer 2017;123:759–68. [PubMed] DOI:10.1002/cncr.30421
- Ho MY, Mackey JR. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag Res 2014;6:253–9. [PubMed] DOI:10.2147/cmar.s40601
- Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract 2016;22:702–16. [PubMed] DOI:10.1177/1078155215625459
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663–71. [PubMed] DOI:10.1056/NEJMoa0707056
- Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol 2009;27:1177–83. [PubMed] DOI:10.1200/jco.2008.18.4028
- Nabholtz JM, Mackey JR, Smylie M, et al. Phase II study of docetaxel, doxorubicin, and cyclophosphamide as first-line chemotherapy for metastatic breast cancer. J Clin Oncol 2001;19:314–21. [PubMed] DOI:10.1200/jco.2001.19.2.314
- Nabholtz JM, Falkson C, Campos D, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 2003;21:968–75. [PubMed] DOI:10.1200/jco.2003.04.040
- Sparano JA, O’Neill A, Schaefer PL, et al. Phase II trial of doxorubicin and docetaxel plus granulocyte colony-stimulating factor in metastatic breast cancer: Eastern Cooperative Oncology Group Study E1196. J Clin Oncol 2000;18:2369–77. [PubMed] DOI:10.1200/jco.2000.18.12.2369
- Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 2013;14:72–80. [PubMed] DOI:10.1016/s1470-2045(12)70525-9
- Swain SM, Jeong JH, Geyer CE Jr. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053–65. [PubMed] DOI:10.1056/NEJMoa0909638
- Mackey JR, Pieńkowski T, Crown J, et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol 2016;27:1041–7. [PubMed] DOI:10.1093/annonc/mdw098
- Kim SB, Kok YT, Thuan TV, et al. Safety results of docetaxel-(Taxotere®)-based chemotherapy in early breast cancer patients of Asia-Pacific Region: Asia-Pacific Breast Initiative II. J Breast Cancer 2015;18:356–64. [PubMed] DOI:10.4048/jbc.2015.18.4.356
- Kim SB, Sayeed A, Villalon AH, et al. Docetaxel-based adjuvant therapy for breast cancer patients in Asia-Pacific region: Results from 5 years follow-up on Asia-Pacific Breast Initiative-I. Asia Pac J Clin Oncol 2016;12:125–32. [PubMed] DOI:10.1111/ajco.12454
- Kim SB, Sayeed A, Villalon AH, et al. Safety analysis of adjuvant chemotherapy with docetaxel administered with or without anthracyclines to early stage breast cancer patients: Combined results from the Asia-Pacific Breast Initiatives I and II. Asian Pac J Cancer Prev 2016;17:697–702. [PubMed] DOI:10.7314/APJCP.2016.17.2.697
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. [PubMed] DOI:10.1200/jco.2009.25.6529
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [PubMed] DOI:10.1200/jco.2013.50.9984
- De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 2008;26:44–53. [PubMed] DOI:10.1200/jco.2007.11.3787
- Shao N, Wang S, Yao C, et al. Sequential versus concurrent anthracyclines and taxanes as adjuvant chemotherapy of early breast cancer: a meta-analysis of phase III randomized control trials. Breast 2012;21:389–93. [PubMed] DOI:10.1016/j.breast.2012.03.011
- Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017;35:2647–55. [PubMed] DOI:10.1200/JCO.2016.71.4147
- de Boer RH, Chan A, Tran B, et al. Use of non-anthracycline regimens in early stage breast cancer in Australia. Asia Pac J Clin Oncol 2011;7:4–10. [PubMed] DOI:10.1111/j.1743-7563.2010.01353.x
- Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist 2004;9:606–16. [PubMed] DOI:10.1634/theoncologist.9-6-606
- Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol 1994;12:888–94. [PubMed] DOI:10.1200/JCO.1994.12.5.888
- Esteva FJ. The current status of docetaxel for metastatic breast cancer. Oncology (Williston Park) 2002;16 Suppl 6:17–26. [PubMed]
- O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10 Suppl 3:20–9. [PubMed] DOI:10.1634/theoncologist.10-90003-20

