Limited energy parametrial resection/dissection during modified laparoscopic nerve-sparing radical hysterectomy
Introduction
The surgical technique of nerve-sparing radical hysterectomy (NSRH) was developed by Japanese researchers in the 1960s (1) and it was classified as type C1 in the new Querleu-Morrow classification system for radical hysterectomy (2). In related anatomical studies, many researchers have suggested that even though pelvic autonomic nerve structures are complex, they mostly travel in a thin sagittal tissue plane dorsal/inferior to the ureter, also known as the “pelvic nerve plane” (3-6). Based on the above-mentioned studies, the Cancer Hospital of the Chinese Academy of Medical Sciences (CAMS) proposed the procedure of “nerve plane-sparing radical hysterectomy” (NPSRH) in 2011 (7). The key point is that the nerve plane dorsal to the ureter is preserved as a whole to simplify the procedure. NPSRH has been demonstrated to be a feasible and safe procedure either via laparotomy or laparoscopy, and can significantly reduce the recovery time of postoperative bladder function (7-9). Even though, certain proportion of patients undergoing laparoscopic NPSRH (NPS-LRH) experience delay in bladder function recovery, using bipolar coagulation in parametrial management (10) because of the nerve thermal damage.
The commonly used bipolar energy devices in laparoscopic surgery can cause nerve thermal damage (11), because its working temperature is as high as 400 ºC, and thermal spread can be up to 6 mm. So bipolar is usually called as “high energy electronic devices”, while ultrasonic scalpel is a kind of instrument relying on mechanical energy to denature the proteins in vessel walls, and thermal spread is only in 1 mm. It produces minimal thermal spread and is considered a kind of limited energy device. Vascular clip is more and more used in recent years because of its non energy and reliable vascular sealing advantages. To further improve the NPS-LRH technique, we employed limited energy parametrial resection/dissection (LEPRD) in pilot cases to reduce thermal injury of the autonomic nerves. An ultrasonic scalpel with very low thermal spread was used to dissect the vessels, then the vessels are secured with vascular clips instead of a bipolar coagulation device. We will describe the LEPRD method and the postoperative outcome.
Materials and methods
Patient characteristics
From July 2012 to January 2016, 257 cervical cancer patients underwent NPS-LRH at the Department of Gynecologic Oncology, Cancer Hospital of CAMS. NPS-LRH was performed by a same surgical team. From 2012 to 2015, electric surgical instruments such as ultrasonic scalpel combining bipolar were used in 163 patients as a kind of common technique. From 2015 to 2016, we tried to use the ultrasonic scalpel to separate the parametrial blood vessels, and then clip them with vascular clips in 94 patients, among which, 65 patients were successful and the left 29 patients were failed and follewed by bipolar coagulation as the remedy. The patients’ data were retrospectively analyzed. This study was approved by the Hospital Ethics Committee. The disease ranged from International Federation of Gynecology and Obstetrics (FIGO) stage IB to IIA. Neoadjuvant chemotherapy (NACT) was given to selected patients with bulky disease (stage IB2 and IIA2). Paclitaxel combined with platinum (cisplatin or carboplatin) was used for 1 or 2 cycles as NACT. Patients who were found to have voiding dysfunction on a preoperative evaluation were excluded from this study.
Procedure of NPSRH
The main difference between NPSRH and NSRH is the preservation of the entire autonomic nerve plane (mesoureter) inferior to the ureter which extends from the midpelvis to the bladder. It does not require the pelvic autonomic nerve fibers to be dissected off from the ureter or the parametrial vasculature. Our previous study has demonstrated this simplified NSRH method results in shorter operative time and less blood loss (9). In the study, NPSRH was performed laparoscopically (NPS-LRH) in all patients.
Iliac vessels were skeletonized after pelvic lymph node dissection. The paravesical and pararectal spaces were fully developed. The uterine artery and the superficial uterine vein were each divided at their origins. All parametrial lymphatic tissues surrounding the deep uterine vein (DUV) were removed, but the original part of DUV which is close to the internal iliac vein and the pelvic splanchnic nerves (PSNs) beneath it were preserved (Figure 1A). The ureter attached to the posterior leaf of the broad ligament and the dorsal mesentery of the ureter [the proximal part of the nerve plane, containing the hypogastric nerve (HN) bundles] were separated laterally. In the site medial to the ureter mesentery, Okabayashi’s space (the pararectal medial space) (5) was developed. The peritoneum of the Douglas pouch was excised, and the rectum was pushed down. The uterosacral ligament was divided via Okabayashi’s space (Figure 1B). The vesicouterine fold of the peritoneum was excised, and the bladder was pushed down to the level of the upper 1/3 of the vagina. Then, the paravaginal space was developed. The ureter was freed and retracted laterally. Between Okabayashi’s space and the paravaginal space, at the medial side of the ureteral dorsal nerve plane [the distal part contains the bladder branch of inferior hypogastric plexus (IHP)], the DUV and the middle and inferior vesical veins (both in the deep layer of the vesicocervical ligament) were carefully dissected and divided, respectively (Figure 1C, D). The bladder branch was protected (Figure 2). Next, the paravaginal tissues were divided, at which level the uterus could be removed, and the nerve plane could be preserved completely (Figure 3).



Use of energy devices
During the limited energy dissection procedure, an ultrasonic scalpel (Johnson & Johnson, New Brunswick, USA) was used for parametrium tissue separation. The uterine artery, DUV and middle and inferior vesical veins were skeletonized and then occluded with vascular clips ( Figure 1C, D). Based on the condition of skeletonized vessels, appropriate vascular clips were selected. Completely skeletonized blood vessels were occluded with Hem-o-lok polymer ligating clips (WECK, Research Triangle Park, NC, USA). Non-skeletonized blood vessels, vessels failed to be occluded with Hem-o-lok clips and the venous plexus can be occluded with laparoscopic titanium clips (Johnson & Johnson). The clipped vessels were divided by the ultrasonic scalpel. In contrast, the routine method we used was to seal the vessels with a bipolar energy device, a BiClamp forceps (Erbe Elektromedizin GmbH, Tuebingen, Germany), after which the vessels were divided using an ultrasonic scalpel. In the cases managed with a limited energy device, BiClamp forceps was an alternative if the vessels failed to be occluded.
Bladder function evaluation
Drainage of urine from the bladder through an indwelling Foley catheter continued in all patients after the operation. On the 7th postoperative day, the Foley catheter was removed. The postvoid residual urine volume (PVR) after spontaneous voiding was measured by ultrasond. The subsequent catheterization was stopped when the patient met all the standards as follows: 1) a sensation of bladder filling; 2) satisfaction of micturition; and 3) PVR<100 mL. The Foley catheter should be kept open until the above criteria were met. The duration of catheterization was recorded. Long-term bladder function was surveyed using a bladder function questionnaire designed specifically for radical hysterectomy patients, concerning the happening of frequent urination, urinary urgency, urinary retention, urinary incontinence, difficult voiding and long-term voiding dysfunction.
Case evaluation
According to the use of different energy devices, the patients were divided into the LEPRD group, the combined modality group and the bipolar group. The age, body mass index (BMI), tumor characteristics and adjuvant therapy of patients in the three groups were documented and compared. The operative time, amount of blood loss, postoperative complications and the length of hospital stay were compared to evaluate the safety of the procedure. Short- and long-term bladder functions were evaluated as surrogate markers for the effectiveness of the autonomic nerve preservation. The short-term bladder function parameters included sensation of bladder filling, self-satisfaction with conscious voiding and post void residual less than 100 mL (9). Long-term bladder function is evaluated via questionnaire and phone follow-up one year after surgery. The questionnaire designed for this study is derived from the Urogenital Distress Inventory (UDI) (12) and includes the following items: urinary frequency, urinary urgency, urinary incontinence, urinary retention and difficulty emptying. One positive item indicates long-term bladder dysfunction (11).
Statistical analysis
The SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Categorical data were compared by χ2 test. For an expected value in any category less than 5, the Fisher’s exact test was adapted for Chi-square test. Quantitative data with normal distribution were expressed as x±s and were compared with the t test. P values was two-sided and P<0.05 was considered statistically significant.
Results
A total of 94 patients underwent LEPRD, instead of bipolar coagulation, during NPSRH. Vascular clipping alone was performed in 65 (69.1%) patients (LEPRD group), and vascular clipping followed by bipolar coagulation was performed in the other 29 (30.9%) patients (combined modality group). Bipolar coagulation (bipolar group) without a limited energy device was used in the remaining 163 patients.
Patient characteristics
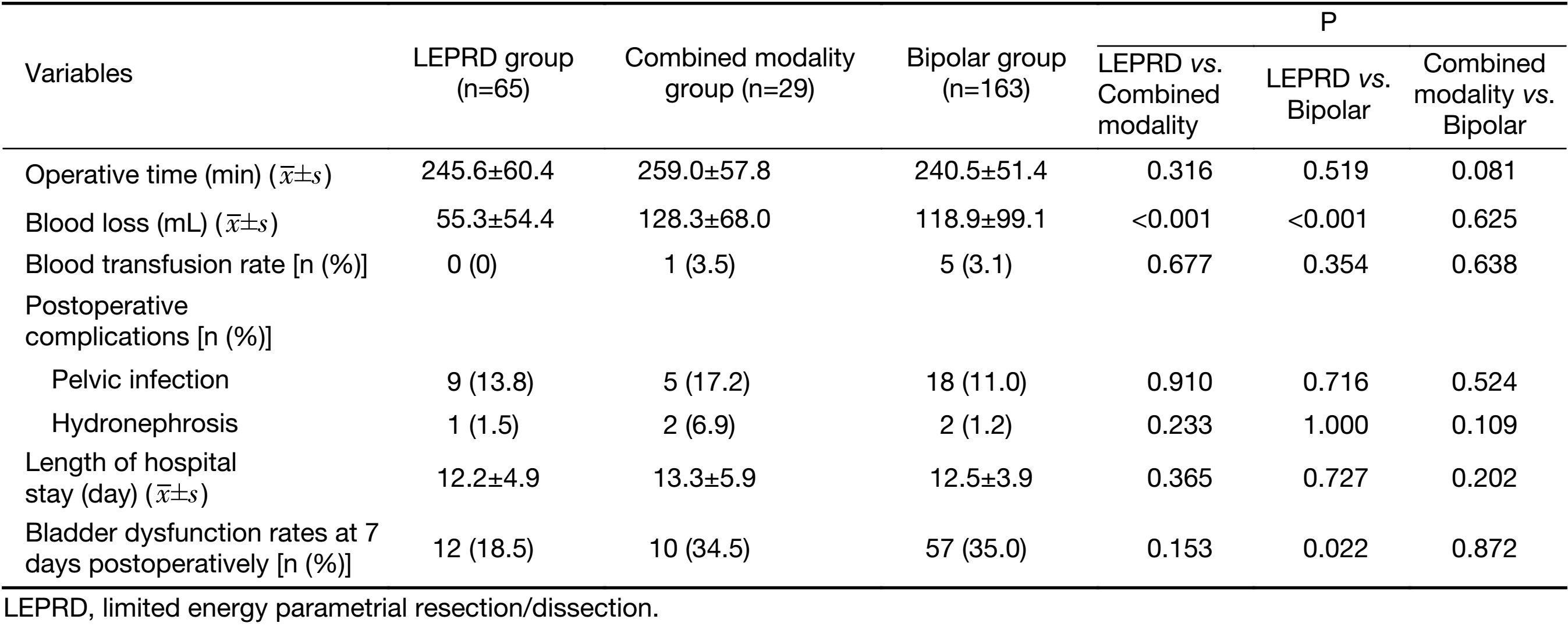
The three groups had no significant difference in age, BMI, International Federation of Gynecology and Obstetrics (FIGO) stage, histologic type, NACT, postoperative radiation, lymphovascular space invasion, depth of invasion and lymph node metastasis. However, the percentage of patients with preoperative tumor size greater than 2 cm was significantly higher in the combined modality group than that in the LEPRD group (P=0.031) (Table 1). LEPRD was attempted in 21 patients where tumor size was >2 cm, and was successful in 10 (47.6%) patients. In the 73 patients where tumor sized was ≤2 cm, LEPRD was successful in 55 (75.3%) patient.

Full table
Clinical assessment
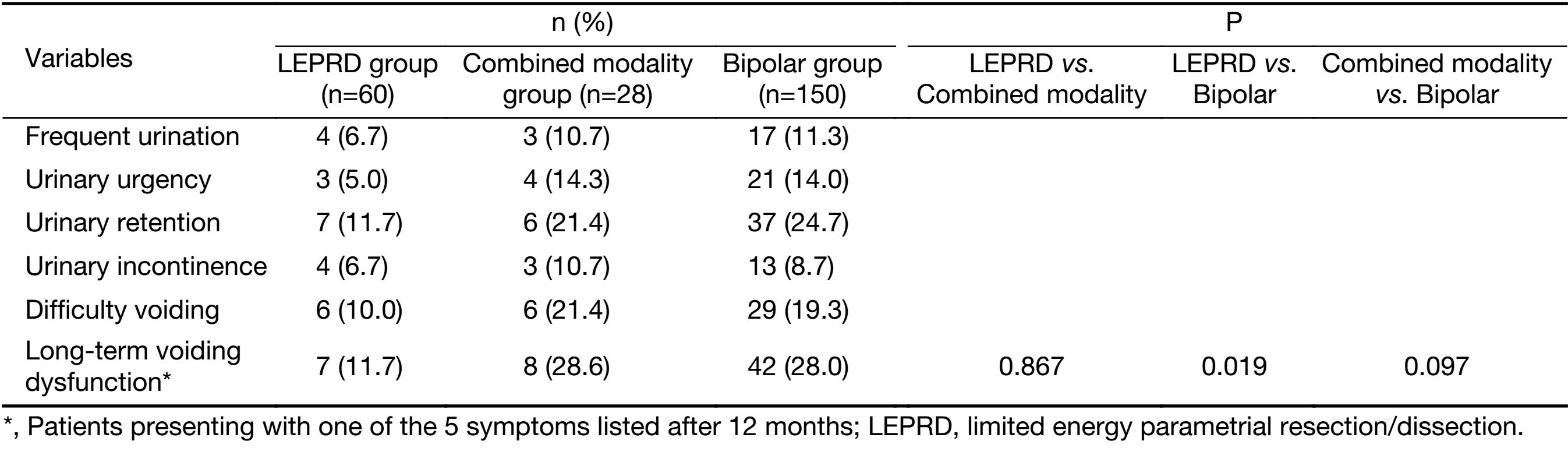
There were no significant differences in operative time, blood transfusion rate, postoperative complications and length of hospital stay among the three groups. The intraoperative blood loss in the LEPRD group was significantly lower than those in the combined modality group or the bipolar group (P<0.001). The proportion of patients who failed to recover bladder function on postoperative day 7 in the LEPRD group (18.5%) was significantly lower than that in the bipolar group (35.0%) (P=0.022) (Table 2). Twelve months after surgery, patients were surveyed via questionnaire; 19 (7.4%) patients were not evaluable due to loss of follow-up (9 patients) or recurrence (10 patients). The incidence of chronic voiding dysfunction was significantly lower in the LEPRD group (11.7%) than in the bipolar group (28.0%) (P=0.019) (Table 3).
Full table
Full table
Discussion
NSRH has evolved as a well-developed technique, and multiple studies have shown that NSRH improves the postoperative quality of life, mainly in improving postoperative bladder, rectal and sexual functions (11,13). Moreover, some studies have shown that NSRH does not compromise the oncologic outcome (14-16), and has been increasingly performed with minimally invasive surgical (MIS) techniques. Compared with conventional laparoscopic radical hysterectomy, laparoscopic NSRH significantly reduces the postoperative time for urinary catheterization and the incidence of voiding dysfunction (10,15,17). Currently, NSRH is considered a type C1 procedure in the Querleu-Morrow classification. However, the description of surgical resection margins for type C1 hysterectomy in the available literature is mainly conceptual and lacks more detailed technical instructions. The complexity of NSRH is reflected in varying surgical techniques and steps described by different reports (14-16). In addition, surgical indication and evaluation method of postoperative autonomic nerve function for NSRH are not standardized. These are factors that hinder the wide clinical application and systematic evaluation of NSRH.
Current studies have established two technical standards for the NSRH procedure (13): 1) The resection margin of the lateral parametrial tissue (cardinal ligament) should extend in depth only to the DUV, and the PSNs dorsal to the DUV should be preserved; and 2) Development of the Okabayashi’s space separates the dorsal parametrial tissue (uterosacral ligaments) from the HN beneath the ureter, and allows preservation of HN while the uterosacral ligaments are transected. These two technical aspects are relatively easy to accomplish. However, preservation of the bladder branches of the IHP, a key step of NRSH, remains difficult (13). Kraima et al. (18) reported that the bladder branches of the IHP have a more diffuse pattern of distribution in the posterior vesico-uterine ligament, often intimately associated with multiple veins. Therefore, it is often difficult to clearly identify and completely preserve all the bladder branches, and we can only rely on the middle or inferior vesical veins as landmarks for approximate locations of the bladder branches. Due to the significant anatomic variability of the middle and inferior vesical veins, the bladder branches can be easily injured with efforts to achieve hemostasis surrounding these veins (19).
In recent years, researchers have gained new insights into the anatomy of the pelvic autonomic nerves (5-8). The HN and the PSNs reside in a thin sagittal tissue plane dorsal to the ureter as a “nerve plane”. It is connected to the ureter, and the nerve fibers run parallel to the ureter. HN is the more dominant nerve bundle in the plane compared to PSNs which is located more dorsally (5-8). Kraima et al. (18) performed an anatomical 3D reconstruction of the IHP bladder branches, showing divergent nerve fibers surrounding the distal ureter within the nerve plane dorsal to the ureter. Therefore, preservation of the dorsal nerve plane can preserve the bladder branches of IHP in a more integral fashion. The above-mentioned studies suggest that the ureter is the key anatomic landmark. If the nerve plane dorsal to the ureter is preserved, it should simplify the procedure and maximize autonomic nerve preservation. Based on the above anatomical findings, we modified the NSRH procedure and proposed the NPSRH procedure (9). The key points of NPSRH include: to identify the dorsal nerve plane of the ureter; to dissect the parametrial tissue via the pelvic avascular spaces (pararectal, paravesical, Okabayashi, and paravaginal spaces) to preserve the nerve plane along with the ureter. The complex procedure of separating the specific structures of the autonomic nerves in the parametrial venous plexus is omitted in the NPSRH, thus simplifying NSRH. We reported previously that NPSRH via the abdominal approach is superior to conventional radical hysterectomy in improved postoperative quality of life, without compromising prognosis (11,20).
In 2014, we reported the preliminary results of NPS-LRH, and confirmed its feasibility (10). Compared to the laparotomy approach, NPSRH performed via the MIS technique allows superior visualization with a magnified view and finer dissection. However, energy devices are commonly used in laparoscopic surgery to secure parametrial vasculature and can cause thermal damage to the adjacent autonomic nervous system. Laparoscopic energy devices include monopolar coagulation, bipolar coagulation and ultrasonic scalpel. The ultrasonic scalpel is an essential instrument for accurate dissection in laparoscopic surgery. It mainly relies on mechanical energy to denature the proteins in vessel walls, resulting in vessel occlusion. The operating temperature of the ultrasonic scalpel is within the tolerance range of normal tissues. It produces minimal thermal spread and is considered a low-energy device. However, it cannot occlude blood vessels more than 3 mm in diameter (21). Bipolar coagulation is the main tool used to secure the parametrial vessels. Electrical current conduction between the two tips produces heat, which in turn causes dehydration and carbonization of blood vessels. The working temperature is as high as 400 ºC, and thermal spread can be up to 6 mm (22). Carlander et al. (4,22) have demonstrated that thermal injury of bipolar coagulation causes irreversible damage to nerve tissues. During NPSRH, we need to manage the uterine and vesical veins on the medial side of the nerve plane. Therefore, the thermal injury of nerves caused by bipolar coagulation is a real concern. For this reason, we routinely use BiClamp forceps to occlude the blood vessels in this area. BiClamp forcep is a new bipolar coagulation device with a biofeedback system that controls the energy output according to the occlusion magnitude of the blood vessel, which can reduce the thermal spread to a certain extent. However, the BiClamp forceps tip is not suitable for accurate separation of blood vessels and nerves; it is only suitable for the occlusion of vessel bundles. Therefore, it cannot avert nerve damage (23).
That is why we investigated the use of vascular clips to secure parametrial blood vessels in an attempt to reduce thermal injury to the nerve plane in NPSRH. Vascular skeletonization with an ultrasound scalpel is an essential step for using vascular clips. We use Hem-o-lok clips to occlude the skeletonized parametrial vessels, including the DUV, the middle and inferior vesical veins. Hem-o-lok clips are non-metallic vascular clips with a lock design on the front to prevent clip slippage. For a single vascular occlusion, these clips are convenient and reliable. They are more commonly used in urologic surgery and are especially suitable for renal vascular pedicle management in laparoscopic nephrectomy (24). Its use has been reported in laparoscopic hysterectomy recently (25). The disadvantage of the Hem-o-lok clip is that its front lock is blunt, and cannot penetrate thick tissue, making it difficult to clip the blood vessels without complete skeletonization (26). Therefore, when Hem-o-lok clip fails, we will supplement with titanium clips. These are the main technical tools used to achieve the total LEPRD. In this study, LEPRD was applied in 94 patients, 69.1% of which were successful (LEPRD group). The remaining 30.9% required additional bipolar coagulation for satisfactory hemostasis (combined modality), and there was a higher percentage of patients with large tumors (>2 cm in diameter). Larger tumors can cause parametrial hypervascularization, difficulty of vascular skeletonization and failure in vascular clipping. Some researchers even suggested that patients with tumor diameters >2 cm are not suitable for NSRH (17). In this study, LEPRD was attempted in 21 patients where tumor size was >2 cm, and was successful in 10 patients (47.6%). In the 73 patients where tumor sized was ≤2 cm, LEPRD was successful in 55 patient (75.3%). Therefore, LEPRD is worth attempting in patients with all tumor sizes, but has a higher success rate in patients with smaller tumors. In addition, vascular clip slippage and displacement are common causes of failure of LEPRD. These common vascular clipping problems need to be improved upon ( 21). This study shows that LEPRD is a safe surgical approach. Blood loss was significantly less in patients in the LEPRD group, and complication rates did not increase. More notably, compared with 163 patients undergoing bipolar coagulation during parametrium dissection, the patients in the LEPRD group had higher rate of short-term bladder function recovery and lower rate long-term voiding dysfunction. However, in cases where the vascular clip failed, the addition of bipolar coagulation reduced the benefit of LEPRD. These facts fully demonstrated that LEPRD can reduce thermal damage and more effectively protect the pelvic autonomic nerves.
One of the limits of this study is that, this is a single-institution retrospective study with a small number of participants. Despite the relatively small sample size of our study, we have demonstrated for the first time, the use of vascular clipping in parametrial dissection during NSRH. LEPRD in NPSRH is a novel technique to further improve and simplify NSRH in the preservation of autonomic nerves. However, we can still improve the consistency, success rate and reproducibility of this novel technique. In addition, the indications of LEPRD still needs to be determined. Long-term follow-up is needed.
Conclusions
LEPRD in NPSRH seems to be a feasible technique that can effectively reduce intraoperative blood loss without increasing surgical complications. In patients with successful LEPRD, the short-term recovery rate of postoperative bladder function significantly increased, and the incidence of long-term urinary dysfunction significantly decreased. These findings suggest that low-energy dissection is an effective approach which can be another choice in the parametrial management of NPS-LRH. However, LEPRD is less successful in patients with larger tumors but is worth attempting.
Acknowledgements
This study was supported by the special fund for Capital City Clinical Specific Application Study (No. Z171100001017115).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kobayahsi T. Abdominal radical hysterectomy with pelvic lymphadenectomy for cancer of cervix. 2nd ed. Tokyo: Nanzando, 1961:86.
- Querleu D, Cibula D, Abu-Rustum NR. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann Surg Oncol 2017;24:3406–12. [PubMed] DOI:10.1245/s10434-017-6031-z
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol 2005;193:7–15. [PubMed] DOI:10.1016/j.ajog.2005.02.108
- Touboul C, Fauconnier A, Zareski E, et al. The lateral infraureteral parametrium: myth or reality?. Am J Obstet Gynecol 2008;199:242. e1–6. [PubMed] DOI:10.1016/j.ajog.2008.04.003
- Yamaguchi K, Kobayashi M, Kato T, et al. Origins and distribution of nerves to the female urinary bladder: new anatomical findings in the sex differences. Clin Anat 2011;24:880–5. [PubMed] DOI:10.1002/ca.21186
- Li H, Jia J, Xiao Y, et al. Anatomical basis of female pelvic cavity for nerve sparing radical hysterectomy. Surg Radiol Anat 2015;37:657–65. [PubMed] DOI:10.1007/s00276-014-1405-4
- Li B, Li W, Sun YC, et al. Nerve plane-sparing radical hysterectomy: a simplified technique of nerve-sparing radical hysterectomy for invasive cervical cancer. Chin Med J (Engl) 2011;124:1807–12. [PubMed]
- Li B, Yao H, Zuo J, et al. Application of laparoscopy in the modified nerve plane-sparing radical hysterectomy of cervical cancer. Zhonghua Zhong Liu Za Zhi (in Chinese) 2014;36:63–8. [PubMed]
- Wang W, Li B, Zuo J, et al. Evaluation of pelvic visceral functions after modified nerve-sparing radical hysterectomy. Chin Med J (Engl) 2014;127:696–701. [PubMed]
- Aoun F, van Velthoven R. Lower urinary tract dysfunction after nerve-sparing radical hysterectomy. Int Urogynecol J 2015;26:947–57. [PubMed] DOI:10.1007/s00192-014-2574-8
- Carlander J, Koch C, Brudin L, et al. Heat production, nerve function, and morphology following nerve close dissection with surgical instruments. World J Surg 2012;36:1361–7. [PubMed] DOI:10.1007/s00268-012-1471-x
- Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res 1994;3:291–306. [PubMed] DOI:10.1007/BF00451721
- van Gent MD, Romijn LM, van Santen KE, et al. Nerve-sparing radical hysterectomy versus conventional radical hysterectomy in early-stage cervical cancer. A systematic review and meta-analysis of survival and quality of life. Maturitas 2016;94:30–8. [PubMed] DOI:10.1016/j.maturitas.2016.08.005
- Basaran D, Dusek L, Majek O, et al. Oncological outcomes of nerve-sparing radical hysterectomy for cervical cancer: a systematic review. Ann Surg Oncol 2015;22:3033–40. [PubMed] DOI:10.1245/s10434-015-4377-7
- Bogani G, Cromi A, Uccella S, et al. Nerve-sparing versus conventional laparoscopic radical hysterectomy: a minimum 12 months’ follow-up study. Int J Gynecol Cancer 2014;24:787–93. [PubMed] DOI:10.1097/IGC.0000000000000110
- van Gent MDJM, Rademaker M, van der Veer JCB, et al. Long-term oncological outcome after conventional radical hysterectomy versus 2 nerve-sparing modalities for early stage cervical cancer. Int J Gynecol Cancer 2017;27:1729–36. [PubMed] DOI:10.1097/IGC.0000000000001067
- Kim HS, Kim TH, Suh DH, et al. Success factors of laparoscopic nerve-sparing radical hysterectomy for preserving bladder function in patients with cervical cancer: a protocol-based prospective cohort study. Ann Surg Oncol 2015;22:1987–95. [PubMed] DOI:10.1245/s10434-014-4197-1
- Kraima AC, Derks M, Smit NN, et al. Careful dissection of the distal ureter is highly important in nerve-sparing radical pelvic surgery: a 3D reconstruction and immunohistochemical characterization of the vesical plexus. Int J Gynecol Cancer 2016;26:959–66. [PubMed] DOI:10.1097/IGC.0000000000000709
- Kyo S, Kato T, Nakayama K. Current concepts and practical techniques of nerve-sparing laparoscopic radical hysterectomy. Eur J Obstet Gynecol Reprod Biol 2016;207:80–8. [PubMed] DOI:10.1016/j.ejogrb.2016.10.033
- Wang W, Li B, Zuo J, et al. Evaluation of postoperative bladder function and prognosis after modified nerve sparing radical hysterectomy. Zhonghua Fu Chan Ke Za Zhi (in Chinese) 2014;49:341–7. [PubMed]
- Harold KL, Pollinger H, Matthews BD, et al. Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the hemostasis of small-, medium-, and large-sized arteries. Surg Endosc 2003;17:1228–30. [PubMed] DOI:10.1007/s00464-002-8833-7
- Carlander J, Johansson K, Lindström S, et al. Comparison of experimental nerve injury caused by ultrasonically activated scalpel and electrosurgery. Br J Surg 2005;92:772–7. [PubMed] DOI:10.1002/bjs.4948
- Li L, Qie MR, Wang XL, et al. BiClamp® forceps was significantly superior to conventional suture ligation in radical abdominal hysterectomy: a retrospective cohort study in 391 cases. Arch Gynecol Obstet 2012;286:457–63. [PubMed] DOI:10.1007/s00404-012-2275-9
- Ping H, Xing NZ, Zhang JH, et al. Application of the Hem-o-lok ligation system in laparoscopic nephrectomy. Surg Endosc 2010;24:1494–7. [PubMed] DOI:10.1007/s00464-009-0782-y
- Lee JE, Kim KG, Lee DO, et al. Ligation of uterine vessels in total laparoscopic hysterectomy using Hem-o-lok clips. Taiwan J Obstet Gynecol 2015;54:8–12. [PubMed] DOI:10.1016/j.tjog.2014.11.001
- Casale P, Pomara G, Simone M, et al. Hem-o-lok clips to control both the artery and the vein during laparoscopic nephrectomy: personal experience and review of the literature. J Endourol 2007;21:915–8. [PubMed] DOI:10.1089/end.2006.0101
